Bioscience
Silkworms provide new spin on sticky molecules
E-selectin is grown in silkworms for the first time, revealing new aspects of its binding dynamics.

KAUST researchers are engineering silkworms to grow the human form of E-selectin, revealing new aspects of its binding dynamics. 2020 KAUST
Silkworms are useful for more than just making strong and absorbent strings of silky thread for the textiles industry. A group of KAUST scientists has now coaxed these grub-like insects into making the human form of E-selectin, a critical adhesion molecule involved in inflammation, cancer and other disease processes.
Working with collaborators at Kyushu University in Japan, biochemist Jasmeen Merzaban and her team engineered silkworms to produce different variants of E-selectin. They then studied how the various adhesion proteins interacted with cells, discovering that there is more to E-selectin’s molecular stickiness than how its terminal binding domain interacts with target molecules.
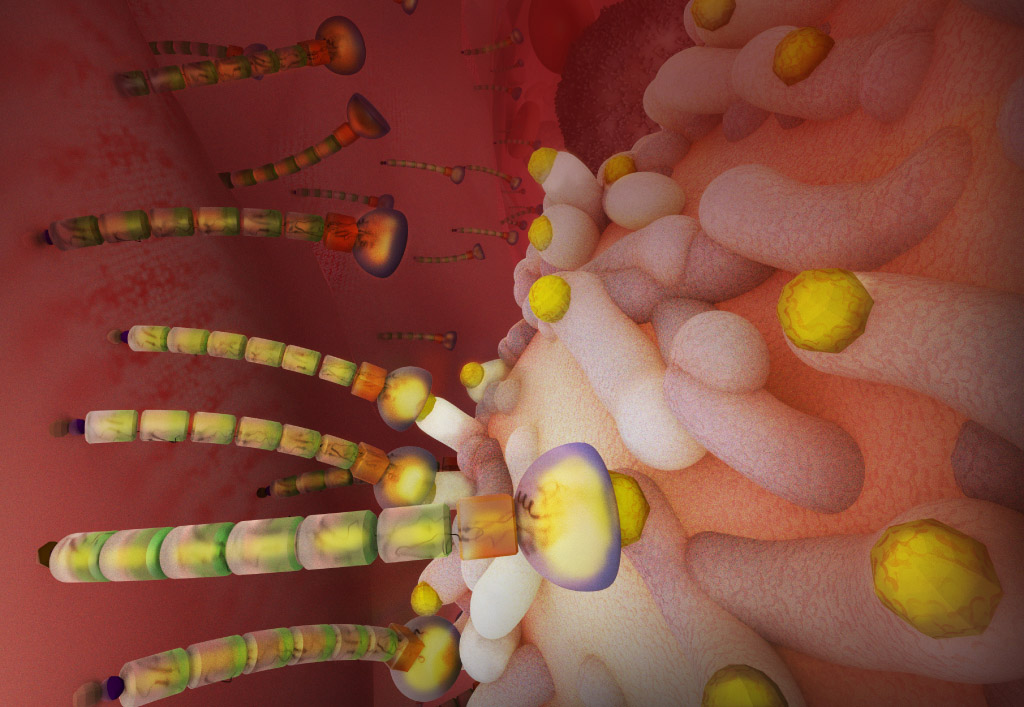
E-selectin is a repetitive region of variable length that extends the protein’s hand to grab cells out of flow. (Featured on the cover of Journal of Biological Chemistry)
© 2020 KAUST; Heno Hwang
For the first time, they have shown that critical for binding is the connecting arm of E-selectin—a repetitive region of variable length (known as the short consensus repeat domain) that extends the protein’s hand to grab cells out of the flow.
Longer armed E-selectins are better at tethering blood stem cells, Merzaban and her colleagues report. But the speed with which E-selectin grabs the cells seems to be controlled only by the protein’s hand and wrist—the lectin and the epidermal growth-factor-like domains, respectively.

First author of the study, Fajr Aleisa, prepares a sample of cells (left) and observes the ability of components of the E-selectin molecule to block adhesion interactions .
© 2020 KAUST
The findings shed light on the natural trafficking of blood components into and out of tissues. They might also explain how rogue cancer cells in the blood get captured at distant organs to seed new tumors throughout the body.
“Evaluating the ability of components of the E-selectin molecule to block adhesion interactions based on our work could generate results with therapeutic implications,” Merzaban says.
She and members of her team are currently exploring the potential for lab-grown E-selectin proteins to serve as decoys in the body. These molecules should get between cancer cells and natural E-selectins found in blood vessels or in the bone marrow, and thereby reduce the rate of metastasis, the main cause of cancer-related death, or prevent tumor cells from hiding away in organs where they are protected from chemotherapy.

Jasmeen Merzaban (back) and Fajr Aleisa discuss the results of their silkworm expression system.
© 2020 KAUST
To manufacture more E-selectin, the team will again rear huge numbers of transgenic creamy white silkworms. Other options for large-scale protein production abound, including the use of Chinese hamster ovary cells and bacterial culture systems. But rodent cells can be expensive and inefficient in making recombinant proteins and bacteria cannot always faithfully replicate aspects of mammalian protein biology.
“The silkworm expression system presents the advantage of producing functional mammalian proteins at large scale with high yields at low cost,” says Fajr Aleisa, the first author of the study and a former Ph.D. student in Merzaban’s lab group.
References
-
Aleisa, F.A., Sakashita, K., Lee, J.M., AbuSamra, D.B., Alwan, B., Nozue, S., Tehseen, M., Hamdan, S.M., Habuchi, S., Kusakabe, T. & Merzaban, J.S. Functional binding of E-selectin to its ligands is enhanced by structural features beyond its lectin domain. Journal of Biological Chemistry 295, 3719-3733 (2020).| article
You might also like

Bioengineering
Bio-inspired network structures for next-generation AI

Bioscience
Robust workflow built for chemical genomic screening
Bioscience
Cell atlas offers clues to how childhood leukemia takes hold
Bioscience
Hidden flexibility in plant communication revealed
Bioscience
Harnessing the unintended epigenetic side effects of genome editing
Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities
Bioscience




