Bioscience
Argonaute proteins help fine-tune gene expression
A protein, with a name reminiscent of legendary Greek sailors, has an unexpected role inside the human nucleus.

A nuclear protein bound to RNA molecules affects chromatin structure and gene expression.
The finding adds to the understanding of a gene silencing pathway called RNA interference (RNAi). The discovery of this pathway in the late 1990s resulted in the 2006 Nobel Prize in Physiology or Medicine being awarded to American scientists, Craig Mello and Andrew Fire.
Argonaute proteins (AGO) that are bound to RNA are known to have a role in RNA interference inside the cytoplasm. But, despite their known presence in the nucleus, the AGO’s role there is not clear.
Now, environmental epigeneticist, Valerio Orlando, together with his team at KAUST and colleagues in Italy and Japan, have uncovered the role of Argonaute proteins in the nucleus, using a combination of genome-wide approaches.
One of the four known Argonaute proteins, AGO1, was found to be present in the nucleus and was more prevalent than other Argonaute proteins on chromatin, the nuclear material that is composed of DNA and its associated network of molecules.
AGO1 was found to bind to “enhancer” regions on DNA, which are responsible for making sure that the right genes are expressed at the right time. This binding was mediated by long nonprotein-coding RNA molecules, called enhancer RNAs.
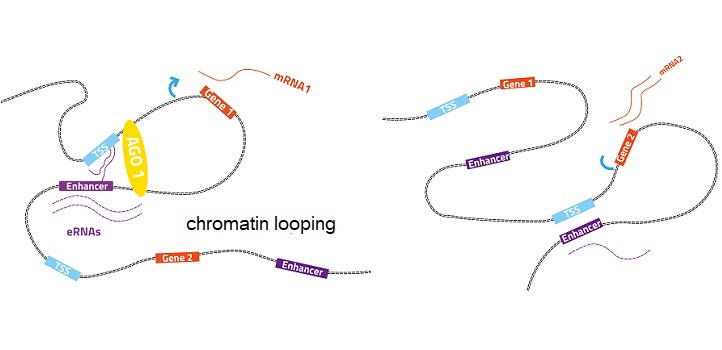
Normal gene expression (left): the enhancer-associated AGO1 helps maintain 3D chromatin organization. Perturbed gene expression (right): turning off AGO1 in human cells changes how chromatin loops interact and perturb gene expression.
© 2019 KAUST; Heno Hwang
Turning off AGO1 in human cells led to extensive changes in the gene expression program. It also led to changes in enhancer RNA levels, in the shape of chromatin, and how its different parts interact.
“The effect of AGO1 on 3D genome architecture was unexpected,” says KAUST epigeneticist Muhammad Shuaib. “Current advances in chromatin structure suggest that changes in its organization can lead to abnormal characteristics in an organism and to diseases,” explains Shuaib. “But the factors involved in establishing and maintaining this architecture, the dynamics of chromatin organization, and its contribution to gene regulation is largely unknown.
Shuaib says that the group’s work not only establishes AGO1 as a new player in the field of 3D chromatin organization, “but also creates a paradigm for future investigations of the nonconventional role of RNA interference components in the nucleus.”
Next, the team plans to study how AGO1 associated with nonprotein-coding RNAs maintains chromatin organization and how its depletion affects the outcome of gene copying inside the nucleus.
References
-
Shuaib, M., Parsi, K., M., Thimma, M., Adroub, S., A., Hideya, K., Seridi, L., Ghosheh, Y., Fort, A., Fallatah, B., Ravasi, T., Carninci, P. & Orlando, V. Nuclear AGO1 regulates gene expression by affecting chromatin architecture in human cells. Cell Systems 9, 1–13 (2019).| article
You might also like

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities

Bioscience
AI speeds up human embryo model research

Bioscience




