Chemical Engineering
A novel catalyst shows promise for better use of carbon dioxide
A new way to utilize a greenhouse gas can enhance the efficiency and stability of carbon dioxide hydrogenation.
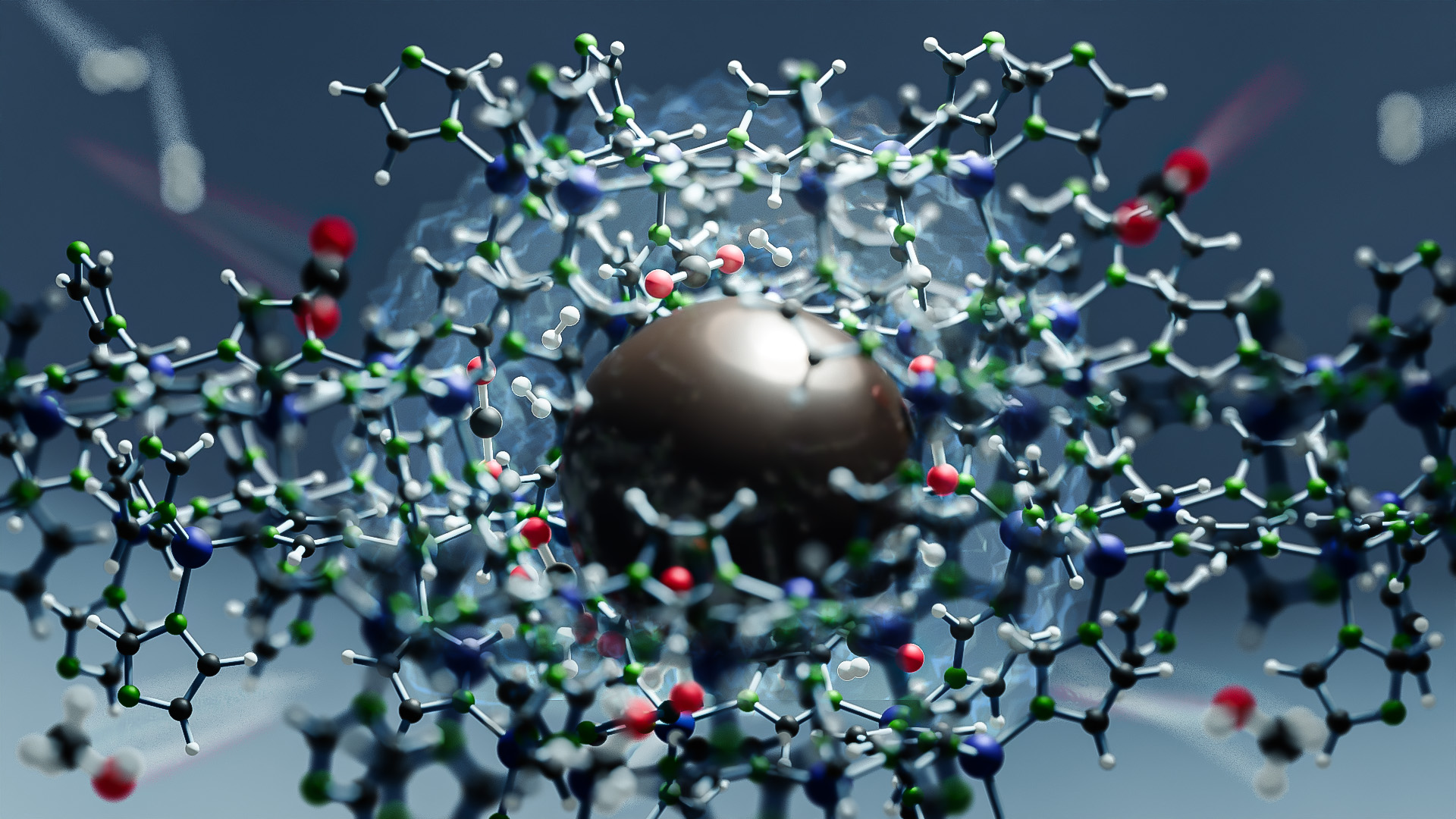
A novel catalyst has been developed that enables carbon dioxide (CO2), an important greenhouse gas, to be converted to methanol under industrially relevant conditions. Developed by a team of international researchers, this discovery presents an efficient and sustainable solution for consuming CO2 and opens up new possibilities for the industrial use of metal organic frameworks (MOFs) in thermal catalysis[1].
Growing concern over climate change has amplified the need to find efficient and sustainable ways to reduce emissions of CO2. One technical solution is to transform this greenhouse gas into useful products such as methanol. To date, the efficiency and stability of the process of hydrogenation of CO2 has been limited by the current suite of catalysts, which tend to be unstable under harsh conditions and low selectivity.
Now, a team has created a new style of catalyst by encapsulating the copper nanoparticles within a zeolitic imidazolate framework-8 (ZIF-8). The team found this unique structure allowed the catalyst to withstand high temperatures and pressures to offer a new option in the field of CO2 hydrogenation.
“We came up with the idea after observing that linker vacancies could generate hydroxyl groups in a thermally stable and hydrophobic MOF; we then proved this using a set of spectroscopic measurements,” explains KAUST research scientist Vijay Velisoju. “This allowed us to generate linker vacancies in the MOF and simultaneously make the copper speciation at sub-nanometric level. These were further stabilized as metal nanoparticles in the MOF network under reaction conditions.”
The copper nanoparticles encapsulated in the ZIF-8 were found to be remarkably stable,” says Velisoju. “But they also had high efficiency and selectivity in the CO2 hydrogenation process: they produced double the amount of methanol than the commercial Cu–Zn–Al catalyst and other MOF-based catalysts. Plus, they remained stable for around six days.
The key to this high activity and stability, the researchers suggest, lies in the uniform distribution of active Cu sites within the ZIF-8. They propose that these sites are kept stable by the framework and hydroxyl groups.
To pinpoint the origin of a material’s catalytic performance and reaction mechanism, we turned to density functional theory (DFT) calculations and advanced in situ characterization tools to better understand the nature of active sites and reaction mechanism elucidation, explains Velisoju.
Our colleagues in KAUST were invaluable in this project,” says Pedro Castaño, the chemical engineer who led the work. “We collaborated with the group of Luigi Cavallo for the calculations, Yu Han for the synchrotron analysis and Mohammed Eddaoudi to double check the MOF structures.
“Our discovery opens several fascinating avenues to make nanocatalysts protected in a highly stable microporous structure with high accessibility as well as creating interphases (in this case Cu-Zn) with outstanding catalytic activity,” says Pedro. This methodology could be further used in other catalytic applications that support the RDIA priorities and the Kingdom’s Vision 2030.
Reference
- Velisoju, V.K., Cerrillo, J.L., Ahmad, R., Mohamed, H.O., Attada, Y., Cheng, Q., Yao, X., Zheng, L., Shekhah, O., Telalovic, S., Narciso, J., Cavallo, L., Han, Y., Eddaoudi, M., Ramos-Fernández, E.V. & Castaño, P. Copper nanoparticles encapsulated in zeolitic imidazolate framework-8 as a stable and selective CO2 hydrogenation catalyst. Nature Communications, 15, 2045 (2024).| article.
You might also like

Chemical Engineering
Urban air pollution goes up in smoke
Chemical Engineering
Rethinking machine learning for frontier science

Chemical Engineering
Magnetic nanoparticles capture microplastics from water

Chemical Engineering
Biogas upgrading goes with a swing

Chemical Engineering
Stronger, lighter, cheaper: a new route to carbon fiber production

Chemical Engineering
Unveiling the role of biomass-burning aerosols in atmospheric reactions

Chemical Engineering
Slashing industrial emissions using a hybrid model approach
Chemical Engineering




