Mechanical Engineering
Sour gas has sweet potential for hydrogen production
Chemical model could help to produce clean fuel from natural gas contaminant.
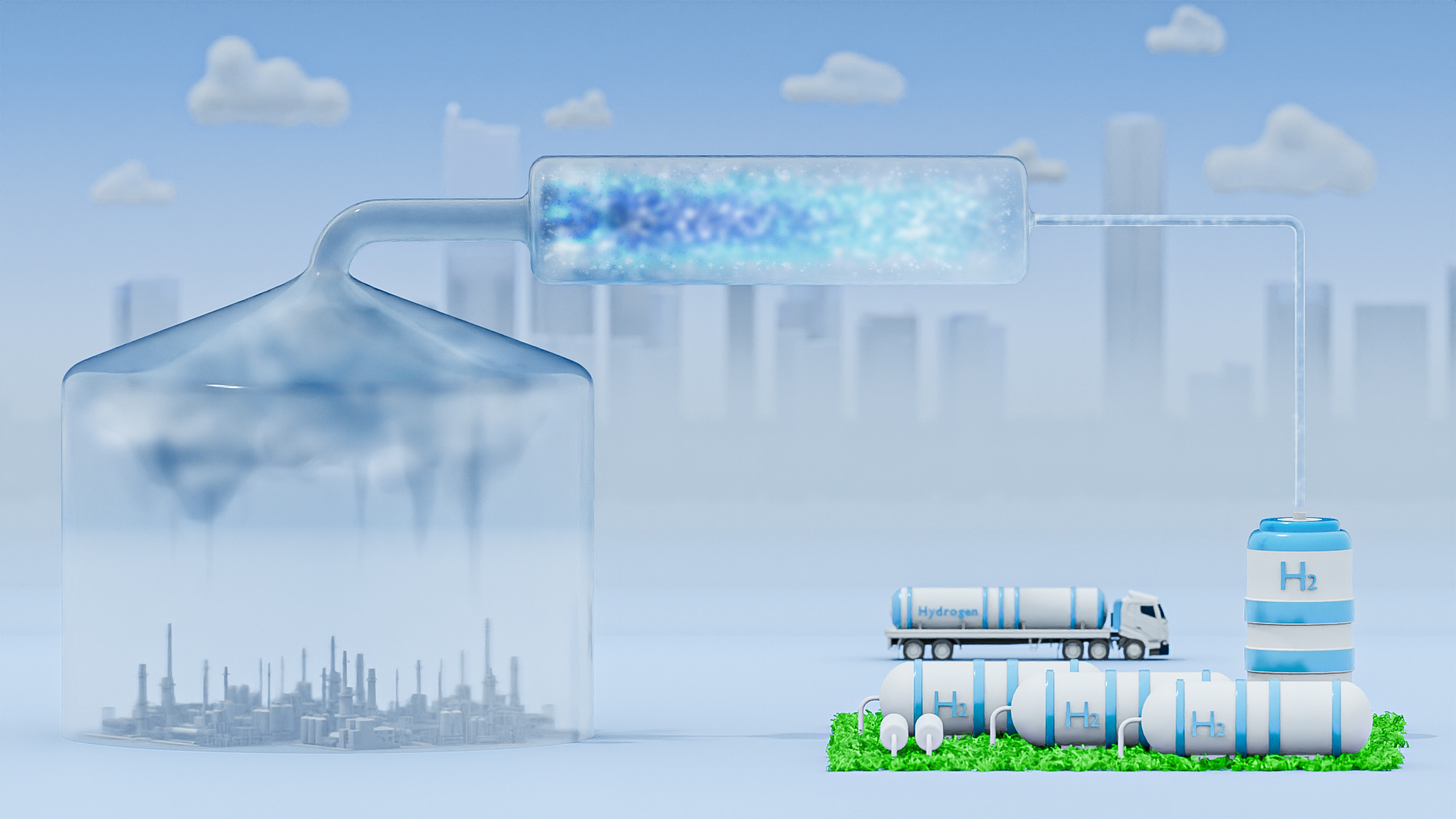
By studying the complex network of chemical reactions between impurities in natural gas, KAUST researchers are paving the way to turn one of those contaminants into clean-burning hydrogen fuel.
Natural gas is mostly methane, a vital fuel and chemical feedstock, but it is often tainted with other molecules. Sour natural gas can contain up to 30% acid gas, a mixture of carbon dioxide (CO2) and toxic hydrogen sulfide (H2S), which must be removed in a process called sweetening before the methane is used.
The most common way to sweeten gas turns H2S into sulfur and water. But H2S could instead act as a source of hydrogen, a green fuel that can reduce the climate-warming CO2 emissions of vehicles and industrial processes. “Acid gas is of low economic value, and commonly present in Saudi gas wells. Hydrogen production from H2S in the presence of CO2 could be a game changer in the Saudi oil and gas industry,” says Aamir Farooq, who co-led the research with Mani Sarathy.
Various chemical strategies could convert H2S into hydrogen, but chemical engineers need to better understand how the interplay between H2S and CO2 would affect the processes. If the molecules react to generate water, for example, it could reduce the efficiency of hydrogen production.
Building on previous studies, the team developed a theoretical model of the reactions that could occur during acid gas processing. This kinetic model predicts how quickly each reaction occurs and which by-products would form[1].
To test their model, the researchers studied these reactions using a low-pressure shock tube. This 18-meter stainless-steel tube uses a pressurized shock wave to rapidly heat mixtures of H2S and CO2 up to 1600 degrees Celsius. Four infrared lasers, each tuned to a characteristic wavelength absorbed by key molecules in the reaction mixture — sulfur dioxide, water, carbon monoxide and CO2 — monitored changes in the mixture over the course of a few milliseconds. The researchers also developed a new thermometric technique, based on changes in the infrared absorption of CO2, to measure the mixture’s temperature.
“Thanks to our novel thermometric technique and high-fidelity modeling, our experimental results match remarkably with our proposed kinetic model, particularly in predicting the onset and rate of formation of key species under various conditions,” says Ali Elkhazraji, the paper’s first author.
One difference was that the experiments did not find any carbonyl sulfide (COS), a precursor of carbon disulfide that was predicted by the model. “Understanding these reactions will help build a more accurate kinetic model, which could be used for maximizing hydrogen production by predicting the product yield, finding optimal operating conditions, and even designing new processes before conducting experiments,” says Qi Wang, the paper’s corresponding author. The team now plans to apply the model to industrial-scale processes.
Reference
- Elkhazraji, A., Wang, Q., Monge-Palacios, M., Zou, J., Alshaarawi, A., Cavazos Sepulvedac, A., Sarathy, S. M. & Farooq, A. Oxidation of hydrogen sulfide and CO2 mixtures: Laser-based multi-speciation and kinetic modelling. Chemical Engineering Journal 486, 150421 (2024).| article
You might also like

Mechanical Engineering
Cracking clean fuel combustion

Mechanical Engineering
Tiny sensor could transform head injury detection
Mechanical Engineering
Electrocatalytic CO2 upcycling excels under pressure
Chemical Engineering
Rethinking machine learning for frontier science

Mechanical Engineering
Falling water forms beautiful fluted films
Mechanical Engineering
Innovative strain sensor design enables extreme sensitivity
Mechanical Engineering
Turbulent flow shows surprise patterns that could help boost efficiency

Mechanical Engineering




