Chemistry | Mechanical Engineering
Squeezing more from carbon dioxide
High-pressure experiments show the way to selectively recycle the greenhouse gas into a particular product.
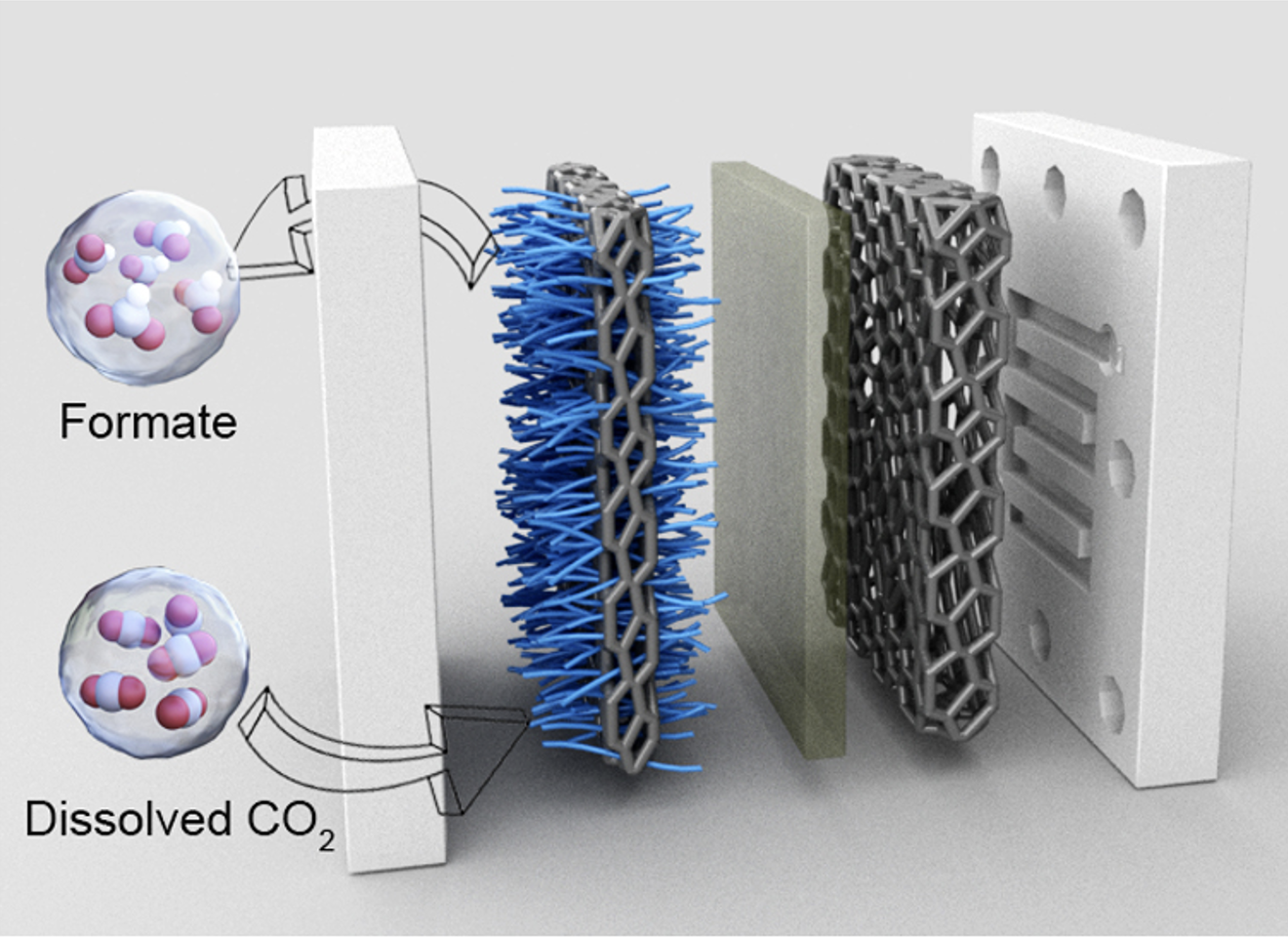
Captured carbon dioxide emissions could be turned into valuable chemicals through a selective process driven by renewable electricity. CO2 can be electrochemically converted into a particular product by running the reaction under high pressure.
“In my research, we use electricity to convert CO2 into fuels,” says Xu Lu, a mechanical engineer from KAUST. The team explored a process called electrochemical CO2 reduction (CO2R), which can turn the waste gas into various potential fuel molecules[1]. Depending on the catalyst used, products including formate, methanol and ethanol can be generated.
Until now, most studies have explored CO2R under ambient pressure conditions. However, in industry, CO2 is typically captured, transported and stored under high pressure. “Since industrial CO2 sources are often pressurized, the direct electrochemical reduction of high-pressure CO2 would be of significant value to industry,” says Liang Huang, a postdoc in Lu’s group.
So Lu, Huang and their collaborators examined what happens when the reaction is run at a pressure of 50 bar, which is more typical of industry conditions. The outcome of the reaction was altered significantly at this pressure.
“To our surprise, we found that catalysts including copper, silver, gold and tin, which exhibited different CO2R selectivities under ambient pressure, all became selective for formate production at high pressure,” Huang says. Converting CO2 into a single product is more useful than generating a mixture.
Understanding reaction processes taking place inside a high-pressure reactor is not trivial because most analytical techniques operate at ambient pressure. To examine the reaction outcome, Lu’s group collaborated with Gaetano Magnotti, who specializes in advanced laser diagnostics and imaging to study high-pressure combustion processes. “We used the high-power system to shoot a laser into our high-pressure electrochemical reactor,” Lu says.
With this setup, the team measured the reactant species appearing on the electrode surface during CO2R. “Together with Edward Sargent’s group from the University of Toronto, we then developed a theoretical model to discover the key factors governing the selectivity shift toward formate,” Huang says.
At high pressure, more CO2 coats the catalyst surface, which boosts the reaction rate and ultimately alters the reaction selectivity, the researchers found. They then showed they could push formate selectivity higher still. “We found that under pressure, a competing reaction called the hydrogen evolution reaction (HER) is the primary factor limiting CO2R selectivity,” Huang says. “We modified our copper electrode surface with a layer capable of inhibiting HER, enhancing formate selectivity.”
The team is now investigating CO2R in different high-pressure reactor designs, including flow cells and membrane electrode assembly (MEA) reactors, exploring their potential value to industry, Huang says.
Reference
- Huang, L., Gao, G., Yang, C., Li, X. Y., Miao, R. K., Xue, Y., Xie, K., Ou, P., Yavuz, C. T., Han,Y., Magnotti, G., Sinton, D., Sargent, E. H. & Lu, X. Pressure dependence in aqueous-based electrochemical CO2 reduction. Nature Communications 14, 2958 (2023).| article
You might also like

Mechanical Engineering
Cracking clean fuel combustion

Chemistry
Turning infrared solar photons into hydrogen fuel

Mechanical Engineering
Tiny sensor could transform head injury detection
Mechanical Engineering
Electrocatalytic CO2 upcycling excels under pressure
Chemical Engineering
Rethinking machine learning for frontier science

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Mechanical Engineering




