Bioscience
A fail-safe mechanism for DNA repair
Single-molecule fluorescent measurements provide fresh insights into a process for keeping errors out of our genomes.

A sequence of events that allows the successful resolution of Holliday junctions at the single-molecule level has been revealed by Samir Hamdan and colleagues at KAUST. The ability to observe the events in real time has enabled the team to learn more about this important mechanism.
Holliday junctions are cross-shaped DNA structures that form when two double-stranded DNA molecules separate into four strands to exchange segments of genetic information. This exchange is essential for correcting errors in DNA introduced during mitotic or meiotic cell division as well as errors caused by environmental mutagens, such as ionizing radiation.
“Holliday junctions are central intermediates in processes rectifying any damage that may affect the integrity and transfer of genetic material to the offspring,” say Mohamed Sobhy and Amer Bralić, the first authors of the study.
Proteins that introduce symmetrically related nicks in strands located across from the Holliday junction restore the integrity of the DNA and allow the recombined strands to separate. Several mechanisms have been shown to mediate this process, known as Holliday junction resolution, and safeguard the completion of DNA repair. Genetic mutations that affect Holliday junction resolution are associated with a predisposition for cancer and genomic instability, which results in higher than normal rates of mutations.
Gap endonuclease I (GEN1) is a cytosolic protein that can bind to Holliday junctions upon breakdown of the nuclear envelope during cell division. Previous studies have shown that GEN1 forms dimers to cleave Holliday junctions, but the exact mechanism by which it ensures the full resolution of Holliday junctions remained unclear.
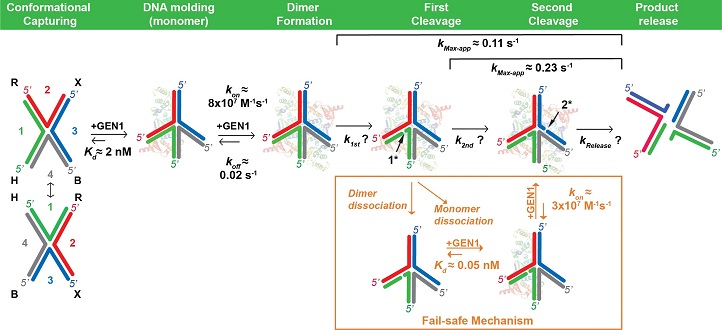
Timeline representation of Holliday Junction resolution by GEN1.
Modified with permission from reference one © 2018 Oxford University Press
Using single-molecule fluorescence resonance energy transfer (smFRET), the authors were able to visualize the interaction of human GEN1 with Holliday junctions in real time. “We saw, for the first time, that the GEN1 monomer distorts the structure of Holliday junctions and remains tightly bound until a GEN1 dimer is formed,” explains Sobhy. Only when the GEN1 dimer has formed, does the cleavage of the Holliday junction take place.
The study also showed that the GEN1 monomer binds to partially resolved (singly cleaved) Holliday junctions with forty times stronger affinity than to intact ones. This is biologically relevant as, according to Bralić, “it suggests that the function of the GEN1 monomer is to safe-guard both intact and partially resolved Holliday Junctions until they are fully resolved just prior to the end of the cell cycle.”
Further research into the mechanisms through which GEN1 cleaves not only Holliday junctions but also other DNA structures may reveal a broader role for GEN1 in preserving the integrity of the genome and affording cancer protection.
References
- Sobhy, M. A., Bralic, A., Raducanu, V. S., Takahashi, M., Tehseen, M., Rashid, F., Zaher, M. & Hamdan, S.M. Resolution of the Holliday junction recombination intermediate by human GEN1 at the single-molecule level. Nucleic Acids Research 47, 4 (2019).| article
You might also like

Bioengineering
Bio-inspired network structures for next-generation AI

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities

Bioscience




