Chemistry
Porous crystals harvest light
An energy transfer process that mimics photosynthesis could help to extract more power from sunlight.
Drawing inspiration from photosynthesis, KAUST researchers have developed a new spin on metal organic frameworks (MOFs) that could help solar cells to gather more energy from the Sun.
A MOF is a kind of porous crystal made from a lattice of metal-based nodes connected by carbon-based linker molecules. MOFs are particularly versatile materials because researchers can easily design and fine-tune their properties by changing the linkers or nodes. MOFs are already being investigated as catalysts and for use in applications, such as gas separation, sensing and storage.
A new MOF developed at KAUST mimics a crucial energy transfer step in photosynthesis, the natural process that plants use to collect light and convert it into chemical energy.
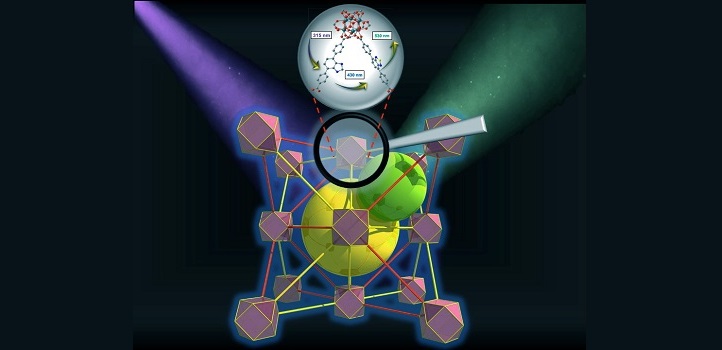
The pale yellow MOF crystals contain 12-coordinated zirconium-based clusters and two different organic linkers: a benzimidazole molecule known as BI, and a thiadiazole called TD. The two linkers were designed not only to have similar size and shape, but most importantly to possess very strong spectral overlap, a key feature for efficient energy transfer processes.
The researchers shone ultraviolet light with a wavelength of 315 nanometers at the MOF. They found that its BI linker absorbed the light and then quickly emitted the energy at a longer wavelength of 430 nanometers, corresponding to blue light. The TD linker efficiently absorbed this blue light, and re-emitted the energy as green light with a wavelength of 530 nanometers.
The researchers monitored the energy transfer process using a technique called time-correlated single-photon counting, which can track the emission of light over incredibly brief timescales. This revealed that the energy transfer process between the two linkers took roughly 100 picoseconds, or one hundred trillionths of a second. “It is challenging to design and synthesize such a light-harvesting system and to observe this fast energy transfer phenomenon,” says team member Jiangtao Jia of KAUST’s Advanced Membranes and Porous Materials Center.
“But thanks to KAUST’s strong research infrastructure, we have one of the best facilities in the world to determine the photoluminescence lifetime to the picosecond timescale,” adds team member Luis Gutieŕrez-Arzaluz.
This enabled the team to ascertain that the energy transfer process had an efficiency of over 90 percent, making it one of the most efficient energy-transfer MOFs to date. “In the future, this deliberate control at the molecular level could pave the way for the design of highly efficient artificial photosynthesis systems based on MOF materials,” says Jia.
References
-
Jia, J., Gutieŕrez-Arzaluz, L., Shekhah, O., Alsadun, N., Czaban-Joźẃiak, J., Zhou, S., Bakr, O.M., Mohammed, O.F. & Eddaoudi, M. Access to highly efficient energy transfer in metal−organic frameworks via mixed linkers approach. Journal of the American Chemical Society 142, 8580–8584 (2020).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering




