Chemistry
A chemical embrace from the perfect host
A molecule with a hollow center proves ideal for separating a common industrial chemical mixture.
KAUST chemists are developing a separation method for the petrochemical industry with high efficiency and low energy consumption.© 2020 KAUST; Heno Hwang
An industrial process that currently consumes vast amounts of energy in petrochemical plants around the globe could be replaced by an alternative process so efficient that it requires no heating or elevated pressure.
Niveen Khashab from KAUST’s Advanced Membranes and Porous Materials Center and her colleagues have developed a new way to separate derivates of benzene, called xylenes.
“Discussions with industry to implement the technology are already underway,” Khashab says.
Xylenes come in three different forms, known as isomers, which differ from each other only in the location of a single carbon atom. Xylenes are used in many large-scale applications, including in polymers, plastics and fibers, and as fuel additives, but many uses rely on just one of the three isomers. Because the isomers are so similar, their physical properties, such as boiling point, are very close. Thus, separating them requires a lot of energy.

Gengwu Zhang performs the separation experiment using two extraction towers.
© 2020 KAUST
“Every year, the global energy costs of separating these isomers using distillation is about 50 gigawatts, enough to power roughly 40 million homes,” says Gengwu Zhang, a postdoc in Khashab’s team and first author of the study. “We wanted to develop a method with high efficiency and low energy consumption to separate and purify these isomers for the petrochemical industry.”
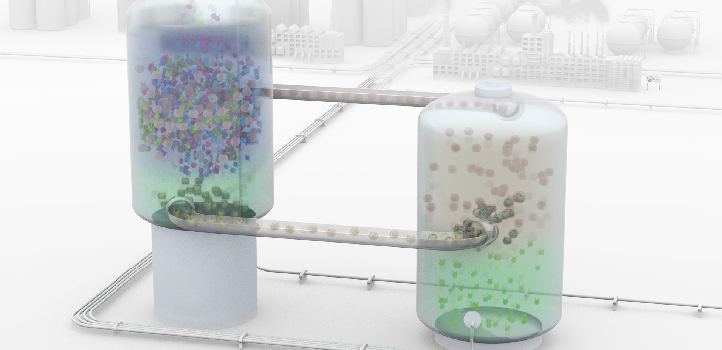
![From a mixture of xylene isomers added to the first extraction tower, cucurbit[7]uril molecules capture only the ortho-xylene isomer. In the second extraction tower, the captured ortho-xylene is released and piped away, while the cucurbit[7]uril is pumped back to the first tank for reuse.](/wp-content/uploads/2023/02/5e6dc769431f994ee943f3e3.jpg)
From a mixture of xylene isomers added to the first extraction tower, cucurbit[7]uril molecules capture only the ortho-xylene isomer. In the second extraction tower, the captured ortho-xylene is released and piped away, while the cucurbit[7]uril is pumped back to the first tank for reuse.
© 2020 Gengwu Zhang
To draw apart the xylene isomers, Khashab and her team used doughnut-shaped molecules called cucurbiturils. The hole in the middle of these molecules can host smaller molecules inside it. The hole in the middle of cucurbit[7]uril is the ideal size for hosting the ortho-isomer of xylene, the team showed. Using a process called liquid-liquid extraction, the team used cucurbit[7]uril to separate ortho-xylene from the other isomers. “We could separate ortho-xylene with more than 92 percent specificity after one extraction cycle,” Zhang says. “Unlike previous methods, our method is performed under ambient temperature and pressure, which means very low energy consumption and easy operation,” he adds.

Gengwu Zhang (left) and Abdul-Hamid Emwas from the KAUST Core Labs discuss the results of the nuclear magnetic resonance experiment for the separation mechanism.
© 2020 KAUST
The whole process was designed to be readily adopted in the existing plants of petrochemical companies, Khashab explains. “Liquid-liquid extraction towers are already used in industry and so it is relatively easy to incorporate our material into this setup,” she says. In addition, cucurbit[7]uril is inexpensive, commercially available or easily made, and highly stable compared with most porous materials.
“We have already shown that we can separate xylene from commercial oil samples at scales of up to 0.5 liters,” Khashab adds. “We are in touch with Saudi Aramco to take this process to industrial implementation.”
Zhang says that the team is also examining other applications for this separation process.
References
-
Zhang, G., Emwas, A.-H., Shahul Hameed, U.F., Arold, S.T., Yang, P., Chen, A., Xiang, J.-F. & Khashab, N.M. Shape-induced selective separation of ortho-substituted benzene isomers enabled by cucurbit[7]uril host macrocycles. Chem 6, 1082-1096 (2020).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering




