Material Science and Engineering
Polymer composites: taking stock of bonds
Theoretical studies analyze the nature of the bonding relationship between conjugated polymers and carbon-based materials.
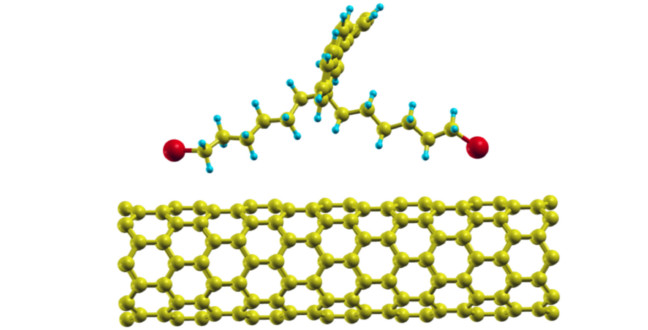

A monomer in contact with a carbon nanotube or graphene and the corresponding densities of states
© Applied Physics Letters/KAUST
Polymers are often reinforced with carbon nanotubes (CNTs) or graphene to produce high performance materials with a wide range of uses, such as electronics, sensors and photovoltaic devices. KAUST researchers are investigating the interactions between the polymers and the CNT components as these bonds affect the optical and electronic properties of the hybrid materials.
Realizing that the binding energy between the polymer and CNTs is particularly significant, Udo Schwingenschlögl and colleagues at KAUST and Bilkent University, Turkey, used computational calculations to study the type of interactions between the two components1.
“It is very difficult to undertake experimental investigations on an atomic level, so we use calculations,” says Schwingenschlögl. “Our aim was to use simulations to predict the quantum-mechanical properties.”
The team used a theoretical method — called density functional theory — to test several different relative orientations of a monomer of a conjugated polymer with either a single walled CNT or graphene. Their analysis shows weak interaction between the components — mostly comprised of van der Waals forces. This supports previous experimental findings that the polymer will interact in a non-covalent fashion and, as a result, will not disrupt the bonding network of the CNT.
Two types of calculations were used by the team to determine the binding energies for different configurations: local density approximation and generalized gradient approximation (GGA). Once van der Waals corrections were included in the GGA model, the researchers found the two methods gave similar binding energies, suggesting that both types of calculations will give accurate results.
Further analysis of the computational results from the monomer-CNT system found no hybridization between the atoms of these two subsystems. Most importantly, the principal features of CNT are preserved, suggesting that its electronic properties would remain unaltered. In studying the monomer-graphene system, the researchers came to similar conclusions, but found stronger binding energies when the backbone of the monomer was aligned with the graphene sheet.
“This is of particular interest because it does not compromise the physical properties of the CNT or graphene, but could improve their solubility and processability,” says Schwingenschlögl.
The study, however, assumes perfect carbon nanotubes. “In reality defects may affect the interaction of these materials with the polymer,” says Schwingenschlögl. His next step is to repeat the experiments while assuming some atomic defects in the CNT or graphene, which would help obtain more practical results.
References
- Jilili, J., Abdurahman, A., Gülseren, O. & Schwingenschlögl, U. Non-covalent functionalization of single wall carbon nanotubes and graphene by a conjugated polymer. Applied Physics Letters 105, 013103 (2014). | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics



