Material Science and Engineering
Elevated design keeps solar stills salt-free
Salt-rejecting microchannels make it easier to turn seawater drinkable using the power of the sun.
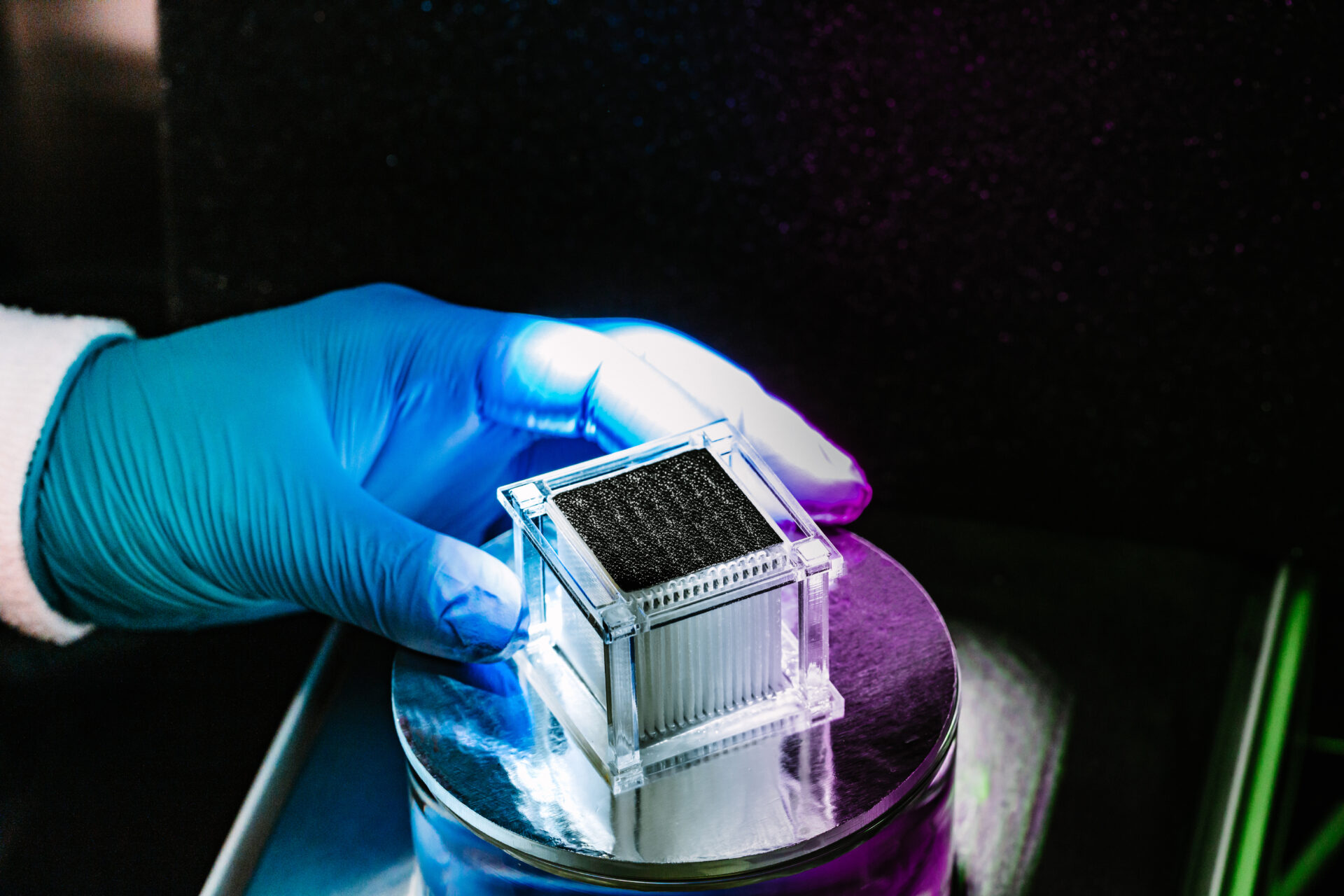
A solar distillation device can purify brine from reverse osmosis plants with over 10 percent salinity, as well as water taken directly from the Red Sea. The technology offers double the freshwater production rate of existing salt-rejection solar stills.
Inspired by the floating solar still in “The Life of Pi” movie, KAUST professor Qiaoqiang Gan has developed several nanomaterials and thermal isolation processes to enhance the evaporation of brackish water into pure steam[1]. In 2016 he launched a startup, Sunny Clean Water, that produces low-cost inflatable stills capable of generating 10-20 liters of fresh water per day.
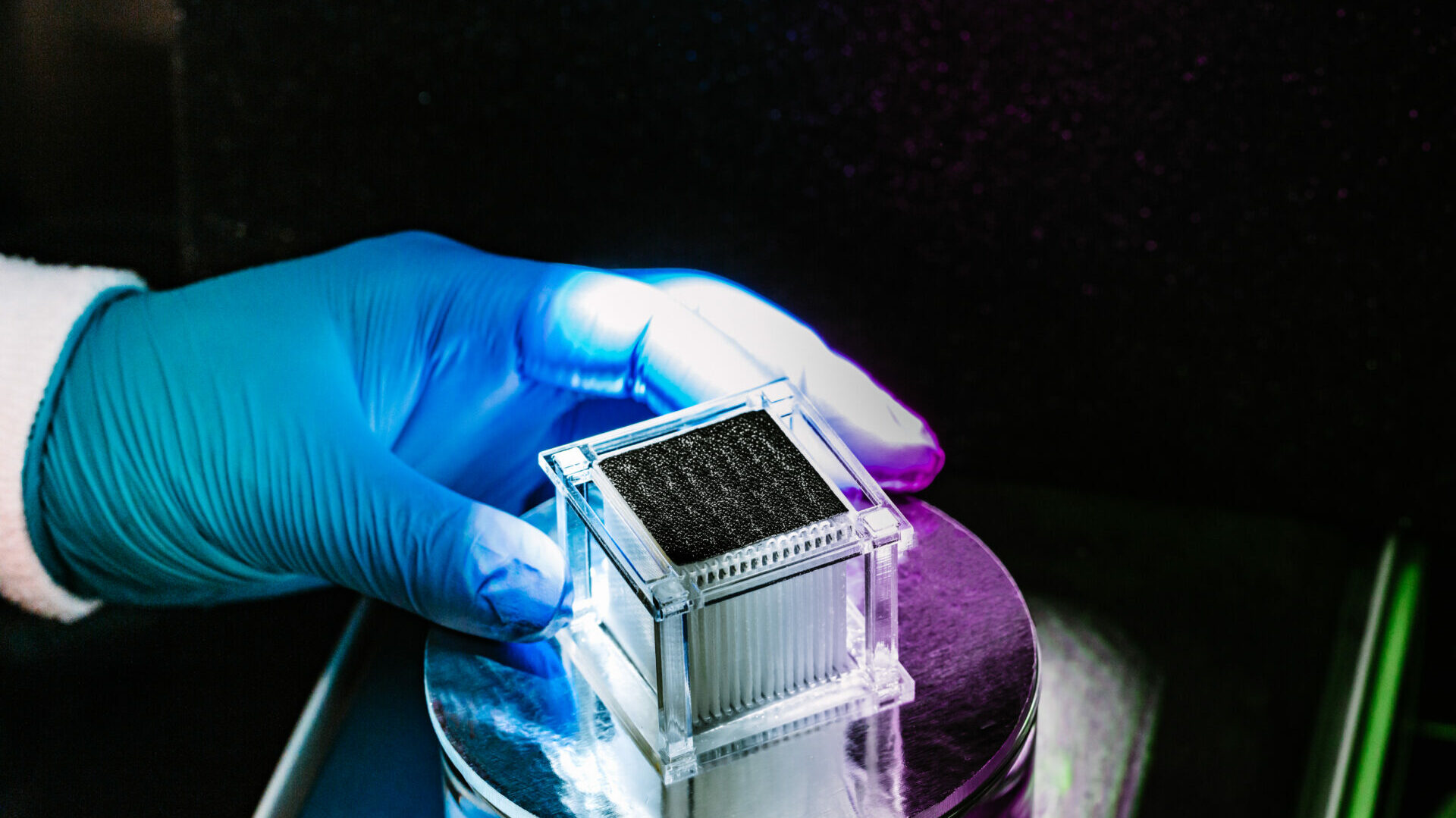
When it comes to seawater purification, however, Gan admits that even his devices have limits. “Over time, you’ll always see salt accumulation on the solar absorbing material — the accumulated salt reflects sunlight and degrades the still’s performance,” he says.

In 2021, Gan joined KAUST and teamed up with fellow KAUST professor Yu Han and researcher Kaijie Yang to improve the efficiency of salt rejection, a strategy that employs techniques such as hydrophobic surfaces or fluid convection to limit mineral buildups.
The team’s new evaporator is a centimeter-scale plastic cube that contains several glass fiber membranes — thin materials normally used for filtration. A horizontally aligned membrane coated with carbon nanotubes acts as a light-absorbing layer on the cube’s upper surface. Underneath it, a series of vertically oriented membranes, or “mass transfer bridges,” separate the solar absorber from the bulk salt water.
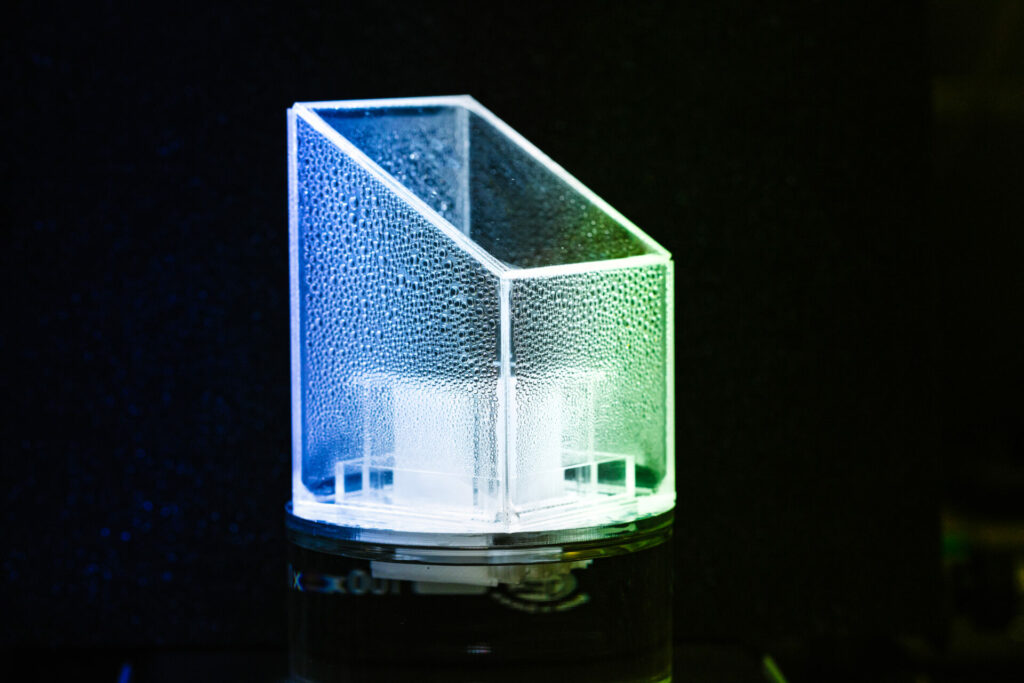
Yang, who conceived the design, explains that the bridges contain hydrophilic microchannels that soak up seawater to the top solar layer for distillation into steam. And when salt accumulation reaches a threshold, the same microchannels transport brine back into the seawater due to the capillary action of concentration gradients.The elevated bridges allow the conductive heat that occurs during salt backflow to flow into the solar still, improving evaporation efficiency. “Other evaporators can realize good salt rejection, but with a short backflow process, there’s a lot of heat energy loss and it impacts water generation rates,” says Yang.

“Our system has the advantage that it can adjust the tradeoff between salt rejection and water generation.”
Testing in both indoor labs and outdoor field stations revealed the solar still could meet the drinking needs of two people daily, with estimated raw material costs of US$50 per square meter.
“We can scale up to a larger architecture by assembling the cubes together,” says Han. “Because this device offers long-term operation without any maintenance, we’re preparing for commercialization.”
Reference
- Yang, K., Pan, T., Dang, S., Gan, Q. & Han, Y. Three-dimensional open architecture enabling salt-rejection solar evaporators with boosted water production efficiency. Nature Communications 13, 6653 (2022).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics




