Environmental Science and Engineering
Bursting bubbles clear the way for desalination
Carbon dioxide bubbles could be a fast and environmentally friendly way to unclog seawater filters and keep the supply of drinking water flowing.
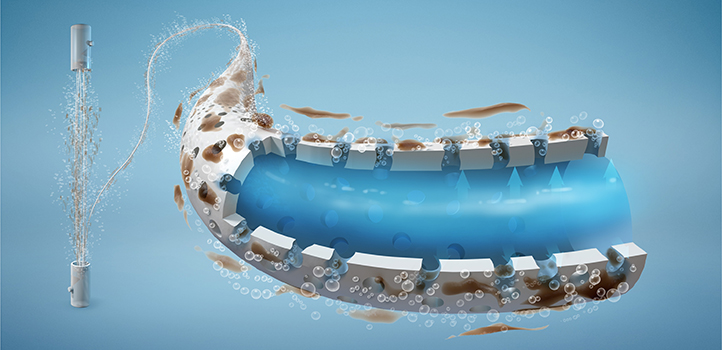
The porous membrane is cleaned without the use of chemicals. © 2019 KAUST; Heno Hwang
Growing populations are increasing the global demand for drinking water, but natural sources of freshwater are shrinking. Extremely arid countries, such as Saudi Arabia, increasingly depend on desalinated seawater.
The most effective desalination begins with ultrafiltration, which removes larger suspended particles and microorganisms. This is followed by the reverse osmosis phase, which draws pure water away from the salty solution through a dense membrane. Ultrafiltration prevents the reverse osmosis membrane from becoming clogged, damaged and unusable, but it also involves a porous membrane that must be regularly cleaned.
“The pretreatment stage is a very important part of seawater desalination,” explains KAUST alum, Mohanned Al Ghamdi, “but cleaning the filters is not easy or environmentally friendly. Repeated use of strong chemicals can damage the membrane beyond repair.”
Al Ghamdi and his Ph.D. supervisor at KAUST, Noreddine Ghaffour, have harnessed the physical and chemical action of carbon dioxide (CO2) bubbles to pluck impurities from ultrafiltration membranes. The technique had been used to wash the surface of reverse osmosis filters, but had not been tested on ultrafiltration membranes to unblock the pores through backwashing.
“We built the whole experiment from scratch,” explains Al Ghamdi. “There was no lab when I started in 2009, so I designed the parts, and we manufactured them at the KAUST workshop.”
After each hour of seawater filtration, a backwash solution is flushed back through the membrane to clear away the gathered impurities. The researchers created their backwash by dissolving CO2 in water until it was suitably saturated. As this solution passes through the membrane, the pressure drops and CO2 bubbles begin to grow, forcefully dislodging the dirt. “We just had to find the ideal starting pressure to enable the most CO2 bubbles to form without damaging the membrane,” explains Al Ghamdi.
In addition to the physical action of the bubbles, CO2 dissolved in water creates carbonic acid, which also helps to break down the dirt. “We were surprised to achieve 100 percent recovery of the filter without the use of chemicals,” says Al Gamdi. “Subsequently, the filter can be used again and again.”Better filter cleaning will reduce the time and expense of replacing damaged membranes, enabling the continuous production of safe drinking water. “We have provided an environmentally friendly cleaning solution that cuts out the use of potentially harmful chemicals,” he adds.

By using carbon dioxide bubbles, scientists are cleaning membrane pores without damaging the membrane.
© Reproduced with permission from reference one © 2019; Xavier Pita
Al Ghamdi explains that the team is now working on improving the technique to reduce cleaning times under different conditions. “It currently takes one minute of backwashing to clean each membrane, but we hope to half that time,” Al Ghamdi says.
Wth increasing demands for drinking water, size is just as important as speed. “We are already scaling up what we have proved to work in the lab through a range of pilot tests,” adds Ghaffour.
References
-
Al-Ghamdi, M. A., Alhadidi, A. & Ghaffour, N. Membrane backwash cleaning using CO2 nucleation. Water Research 165, 114985 (2019).| article
You might also like
Environmental Science and Engineering
Bacteria reveal hidden powers of electricity transfer

Environmental Science and Engineering
Wastewater surveillance tracks spread of antibiotic resistance

Bioscience
Super fungi survive extreme Mars-like environments

Environmental Science and Engineering
Rethinking food systems to restore degraded lands

Environmental Science and Engineering
Combat climate change by eliminating easy targets

Environmental Science and Engineering
Wastewater treatment to fight the spread of antibiotic resistance
Bioscience
Digging into the world of plant-growth-promoting microbes

Bioscience




