Chemistry
Stabilizing ligands make nanoclusters brighter
Large cylindrical ligands increase stability and enhance light emission of silver nanoclusters.
Metal nanoclusters that bear tunable surface ligands could help develop next-generation imaging and photocatalytic approaches, suggests work by KAUST researchers.
Metal nanoclusters that bear tunable surface ligands could help develop next-generation imaging and photocatalytic approaches, suggests work by KAUST researchers.
Metal nanoclusters, usually less than two nanometers in size, exhibit unique physical and chemical characteristics that are useful for a multitude of applications, ranging from catalysis and sensing to imaging and drug delivery. These properties hinge on the size and stability of the nanoclusters. Several ligands have proven effective for stabilizing the nanoclusters and tuning their properties according to specific uses. However, these size-dependent properties remain difficult to harness.
Silver nanoclusters tend to have low stability. Although some of these nanoclusters remain stable over a few days, most disintegrate within minutes, explains Ph.D. student Laila Khalil. This demise highlights the need for stabilizing ligands that can also improve the optical properties of these nanoclusters.
Now, a team led by Niveen Khashab has devised a way to increase stability. They developed sulfur-based ligands with a large cyclic functional group called a pillararene. These ligands can simultaneously stabilize silver nanoclusters. They feature a cylindrical cavity that can accommodate small molecules, or guests, and selectively bind to these guests through noncovalent interactions.

Basem Moosa (left) and Laila Khalil discuss the sulfur-based ligand.
© 2019 KAUST
“We create and synthesize systems that mimic natural designs,” says Khalil to explain why the team decided to produce a macrocyclic thiol ligand. Unlike typical macrocycle-based ligands, such as the hydrophobic and cone-shaped calixarenes, pillararenes are electron-rich cylindrical structures that can be readily modified using various functional groups, which allows them to hold electron-poor and neutral compounds in their cavity. This is expected to widen the range of potential guest molecules and consequently the ability to tailor the properties of the nanoclusters.

Laila Khalil collects the purified ligand (left). The nanocluster solution is treated with a surfactant and UV light to induce a 2,000-fold increase in luminescence.
© 2019 KAUST
The pillararene-functionalized nanoclusters stayed stable for four months when stored in the dark and for up to seven days when exposed to daylight. Their photoluminescence rose 30 times when a neutral amine was used as a guest molecule. Adding the cationic surfactant cetrimonium bromide induced a 2000-fold increase in photoluminescence that was visible to the naked eye, and it also outperformed other atomically precise nanoclusters. The researchers demonstrated that stronger binding between guest molecules and nanoclusters led to a more pronounced increase in photoluminescence. This suggests that the dramatic increase in emission results from host-guest interactions.
This system could have useful biological applications, especially noninvasive deep-tissue imaging,” says Khalil. This will help diagnose diseases, such as skin cancer and brain anomalies.
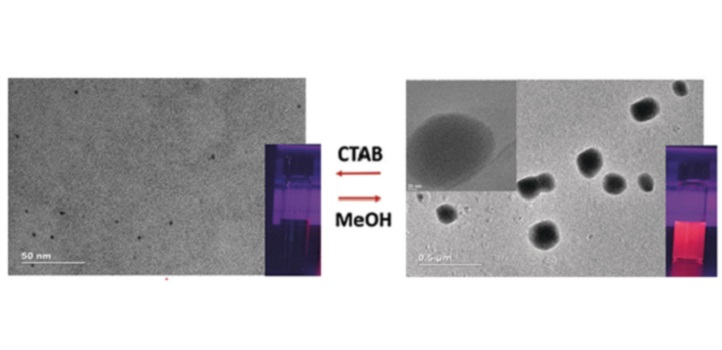
The nanoclusters form round aggregates in the presence of cetrimonium bromide through reversible host-guest interactions.
Reproduced with permission from reference 1 © 2019 Wiley-VCH
References
-
Muhammed, M., Cruz, L. K., Emwas, A.-H., El-Zohry, A., Moosa, B., Muhammed, O.F., Khashab, N.M. Pillar[5]arene stabilized silver nanoclusters: extraordinary stability and luminescence enhancement induced by host-guest interactions. Angewante Chemie International Edition 58, 15665-15670 (2019).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering




