Chemistry
Imperfection is OK for better MOFs
Imaging defects in MOF crystals, and monitoring how they develop, will allow control of defect formation to design better MOFs for many applications.
Perfect crystals are not necessarily the most useful. Defects in the ordered crystalline structure of metal-organic frameworks (MOFs) could tailor these versatile materials for specific applications. KAUST researchers have already developed a pioneering method to image the defects using transmission electron microscopy. They now report that creating specific defects, visualizing them, and investigating their chemical effects takes the exploration of MOFs to new levels of detail and control.
MOFs contain regularly spaced metallic clusters connected by carbon-based organic linker groups. Varying the metals in the clusters and the structure of the linkers creates a huge diversity of MOFs with varying pore networks and different chemical properties. Two of the major applications MOFs are being developed for are for use as catalysts and as highly selective gas adsorption and separation materials.
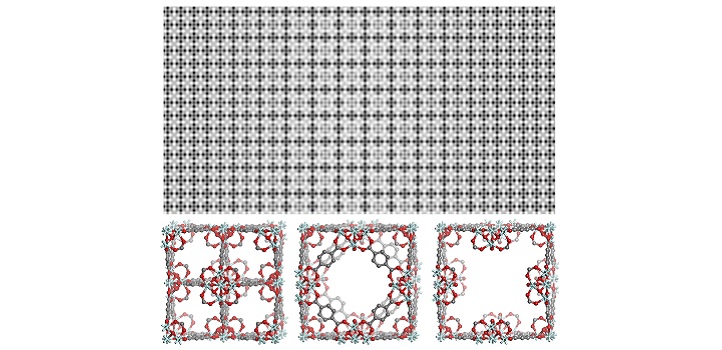
(Top) Processed high-resolution TEM image (top), showing coexistence of various defective structures in MOF UiO-66. (Bottom) Crystallographic models of three types of identified defects in MOF UiO-66.
© 2019 KAUST
MOFs are one of the hottest areas of chemical research, and KAUST scientists are hard at work to remain in the forefront. The latest advance builds on a long record of discoveries and has involved three KAUST research centers, the KAUST Core labs and collaborators in China and the UK.
“The biggest surprise we are revealing is that there are diverse defects in almost all MOFs, even those that were previously considered to be perfect,” says researcher, Yu Han of the KAUST Advanced Membranes and Porous Materials Center.
Han explains that investigating the defects is challenging because MOF crystals are fragile and easily damaged by the electron beams used in conventional electron microscopy. The KAUST team has overcome this problem by using a highly sensitive electron-counting camera, combined with a suite of specially designed image processing methods.
This new ability to peer directly into a MOF at a high level of resolution reveals that two types of defects can coexist, due to missing metallic clusters and missing linkers. “Such details could not be seen prior to our work,” says Han.
The researchers also explored creating defects in MOFs with chemical treatment and monitoring how the pattern of defects develops. This demonstrates the potential to fine tune the defects to manipulate the chemical properties of a MOF.
The KAUST team has demonstrated the power of this strategy by finding that a specific MOF with missing cluster defects is more catalytically active than one with missing linker defects.
The researchers are now working to further refine their imaging technique and to apply it to larger crystals. “We hope to disclose more unknowns about MOFs in order to optimize their applications,” says Han.
References
- Liu, L., Chen, Z., Wang, J., Zhang, D., Zhu, Y., LIng, S., Huang, K.-W., Belmabkhout, Y., Adil, K., Zhang, Y., Slater, B., Eddaoudi, M. & Han, Y. Imaging defects and their evolution in a metal-organic framework at sub-unit-cell resolution. Nature Chemistry 11, 622–628 (2019).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering




