Chemistry
Crystals that clean natural gas
A metal-organic framework that selectively removes impurities from natural gas could allow greater use of this cleaner fossil fuel.
Removing the troublesome impurities of hydrogen sulfide (H2S) and carbon dioxide (CO2) from natural gas could become simpler and more effective using a metal-organic framework (MOF) developed at KAUST.
Upgrading natural gas in this way could help Saudi Arabia to make greater and cleaner use of its abundant natural gas supplies, which can contain high levels of these two impurities. The technology could also promote increased use of natural gas and other industrial gases containing H2S and CO2 worldwide, to reap potentially large environmental and economic benefits.
Natural gas is largely composed of methane (CH4) and smaller quantities of other useful hydrocarbons, together with some impurities. Once stripped of contaminants, natural gas burns much more cleanly than other fossil fuels: it emits no sooty particulates as well as less CO2 and polluting oxides of nitrogen and sulfur.
This advance will support Saudi Arabia’s Vision 2030 program, a major initiative, aimed at reducing the Kingdom’s dependence on oil and developing new environmentally sustainable technologies. This drive includes the goal to source 70 percent of energy from natural gas.
“Meeting this challenging target will require enhanced use of sources of natural gas that initially contain significant levels of H2S and CO2,” says Youssef Belmabkhout of the KAUST team.
MOFs contain metal ions or metal clusters held together by carbon-based organic chemical groups known as linkers. Rearranging different linker and inorganic molecular building blocks fine-tunes the size and chemical properties of the pore system in MOFs and enables them to perform many useful functions.
“The challenge we met in this work was to develop a fluorine-containing MOF with pores that allow equally selective adsorption of H2S and CO2 from the natural gas stream,” Belmabkhout explains.
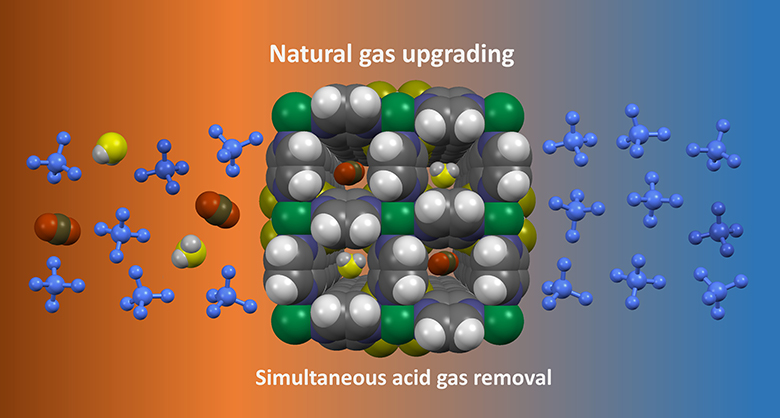
This tailor-made MOF adsorbent removes hydrogen sulfide (yellow and grey) and carbon dioxide (black and red) contaminants from the natural gas stream for a pure methane (blue) product (right side).
© 2018 KAUST
The research was performed by a group in the KAUST Advanced Membranes & Porous Materials Center, led by Professor Mohamed Eddaoudi. This center has a long history of developing MOF adsorbents for many applications, including catalysis, gas storage, gas sensing and gas separation.
“Recent advancements in MOF chemistry at KAUST have permitted the design and construction of various MOF platforms with the potential to address many challenges pertaining to energy security and environmental sustainability,” says Eddaoudi.
Much of the research on upgrading natural gas was funded by the Saudi national petroleum and natural gas company Aramco. “The interest of Aramco certainly corroborates the importance of this work for the kingdom,” adds Eddaoudi.
A new project with Aramco is also underway; it will investigate scaling up the procedure in preparation for commercial exploitation. Further research on optimizing the chemical features of the MOF is also being discussed with other industrial partners.
“This is about much more than chemistry,” Belmabkhout emphasizes, “It is about combining chemistry, chemical and process engineering, physics and computation together with industrial partners to advance the economic use of a natural resource.”
References
- Belmabkhout, Y., Bhatt, P.M., Adil, K., Pillai, R.S., Cadiau, A., Shkurenk, A., Maurin, G., Gongping, L., Koros, W. & Eddaoudi, M. Natural gas upgrading using a fluorinated MOF with tuned H2S/CO2 adsorption selectivity. Nature Energy 3, 1059–1066 (2018).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering




