Chemistry
Softly softly approach for tiny catalyst
Researchers reveal a low-impact synthetic strategy for producing supported small nickel nanoparticles – a powerful catalytic form of the metal.
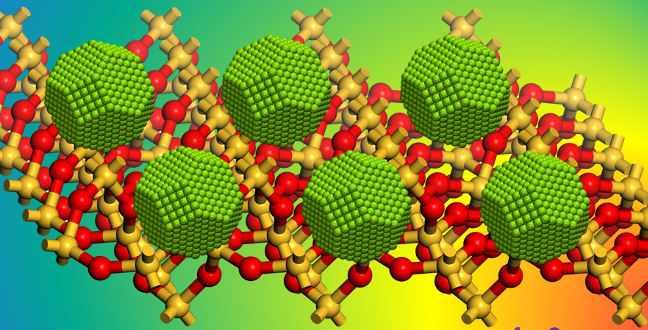
The tiny nickel nanoparticles that the researchers produced can make for an excellent catalyst for many different chemical reactions.
© 2014 KAUST
A gentle synthetic strategy has been devised by chemists at the KAUST Catalysis Center that overcomes some practical obstacles when using nickel as a catalyst.
Nickel is highly popular as a catalyst because it is cheap and can be used for a wide range of applications. As a catalyst it is usually employed in either its molecular complexes, or in the form of nickel nanoparticles supported on an inert substrate. The smaller the nickel nanoparticle, the more efficient the catalysis. But producing nickel nanoparticles of the requisite tiny size is difficult because the nickel nanoparticles tend to aggregate to form clusters. Also, being so small, the particles can be damaged by the high temperatures of the treatment process.
A team lead by Jean-Marie Basset has managed to overcome these challenges to produce small nickel nanoparticles (about one to three nanometers in diameter) that are uniformly distributed on inert oxide surfaces1. The researchers were able to demonstrate the effectiveness of the new tiny catalysts in the dry reforming of methane: a reaction between carbon dioxide and methane to carbon monoxide and hydrogen.
“What is exciting about this method is the very soft control, at very low temperature, of the first step of the catalyst preparation,” said Basset. “It is important to control the elementary steps of this surface reaction because often the nanoparticles used in catalysis are prepared by steps which are not fully understood.”
Using silica and alumina as inert oxide substrates, the team partially dehydroxylated the surfaces so they could control the number of reactive surface groups present. Then they reacted the precursor, Bis (η3 allyl) nickel complex — a complex of nickel with six carbon atoms— with the surface hydroxyl groups in a one-to-one ratio. Both steps were closely controlled by using Infrared Spectroscopy (FTIR) and Solid state NMR to follow the characteristic wavelengths that correspond to the addition or removal of chemical groups.
Finally, the team subjected the surface nickel complexes to hydrogen gas flow at 300 degrees centigrade to produce the silica- and alumina- supported nickel nanoparticles.
The dry reforming of methane, Basset explains, is a very clean and selective reaction when the nanoparticles of nickel catalysts are very small, like the ones produced by his team.
Basset explains that the benefit of the process is two-fold— taking two environmentally harmful greenhouse gases and converting them to two products that are useful for chemical industry.
References
- Li, L., Abou-Hamad, E., Anjum, D. H., Zhou, L., Laveille, P. V., Emsley, L., & Basset, J.-M. Well-defined mono (η 3-allyl) nickel complex [triple bond, length as m-dash] MONi (η 3-C 3 H 5)(M= Si or Al) grafted onto silica or alumina: a molecularly dispersed nickel precursor for syntheses of supported small size nickel nanoparticles. Chemical Communications, 50, 7716-7719 (2014).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



