Environmental Science and Engineering
Probing water for an electrifying cause
An experiment, elegant in its simplicity, helps explain why water becomes electrified when it touches hydrophobic surfaces.

KAUST researchers are delving deeper into water. © 2020 KAUST /en/article/1058/probing-water-for-an-electrifying-cause
KAUST researchers are delving deeper into water. © 2020 KAUST /en/article/1058/probing-water-for-an-electrifying-cause
For over a century, scientists have been puzzled by the electrification of water when it is brought in contact with water-repellent or “hydrophobic” materials, such as paraffin wax, oils, air bubbles and perfluorinated membranes and sheets. Underlying mechanisms remain hotly debated. Now, a team of KAUST engineers has untangled the roles of water, hydrophobicity and environmental factors in this process. This fundamental contribution could support development of better devices for microfluidics and nanofluidics and for generating clean energy.
“Hydrophobic surfaces are quite common,” notes Jamilya Nauruzbayeva, Ph.D. student and lead author of the study. “For instance, polypropylene and perfluorinated pipettes, tubes, coatings and membranes are hydrophobic surfaces used for many basic sciences and engineering applications. Thus, it is important to understand which mechanisms are at play to improve them and develop new ones.”
Himanshu Mishra, who conceived and led this study, says that he has been thinking about this problem for over five years. “Probing the surface of water is an excruciatingly difficult undertaking because the thickness of interfaces is down to the molecular scale, which no experimental techniques can probe unambiguously,” Mishra explains.
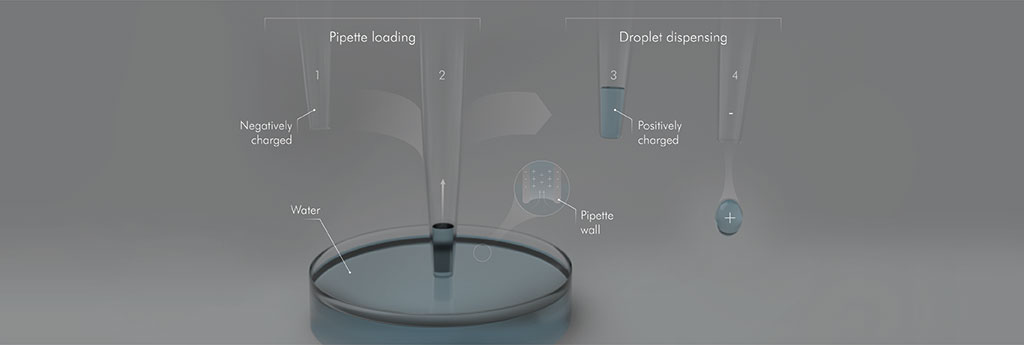
Hydrophobic surfaces (1) are intrinsically negatively charged and so when the hydrophobic capillary (2) draws in water from the (neutral) water reservoir, it selectively attracts cations (i.e., positively charged ions) that populate the electrical double layer (shown in the insert). Thus, when the capillary (3) is pulled out of the water reservoir, it carries water with a net-positive charge leaving behind an equal and opposite charge in the water reservoir. When the water is dispensed (4), hydrophobicity ensures that the entire volume of water is expelled with the excess positive charge.
Reproduced under a Creative Commons 4.0 license from reference one © 2020 Nauruzbayeva et al.; KAUST; Ivan Gromicho
“This is an electrifying subject at water conferences; over the years, through experiment and theory, several competing factors and mechanisms have been proposed,” says Mishra. These include, for instance, the dipolar nature of the water molecule; the instantaneous charge transfer between interfacial water molecules and hydrophobes; the dissolution of atmospheric CO2 in water; and the interfacial accumulation of intrinsic ions of water (i.e., hydroxide and hydronium ions).
Mishra and his students teamed up with Carlos Santamarina to design elemental experiments to unentangle the role of water, its ions and pH, hydrophobicity of surfaces, and environmental factors, such as relative humidity and CO2 content.

(l-r) Jamilya Nauruzbayeva, Himanshu Mishra and Carlos Santamarina discuss the progress or their study.
© 2020 KAUST; Anastasia Serin
Using a parallel plate capacitor, they exposed “pendant” droplets formed from hydrophobic capillaries, which respond to uniform electric fields. The competition between their weight and the electrical force tilted the pendant droplets, which revealed their charge.
Next, they utilized an electrometer—capable of measuring charges down to a few electrons—to measure the charge of the water reservoirs from where the droplets were withdrawn. They discovered that as a water droplet is withdrawn using a hydrophobic capillary, the water reservoir acquires an equal and opposite negative charge. This is not the case when you use a glass capillary.

Jamilya Nauruzbayeva (back) and Adair Gallo delve into the results of their study.
© 2020 KAUST; Anastasia Serin
“From these experimental results, we could deduce that these hydrophobic surfaces carried negative surface charge, even in air, which is quite counterintuitive,” explains Nauruzbayeva. “When the surface is inserted in water, positive ions are attracted toward it and negative ions are repelled. Hydrophobicity ensures that the liquid departs from the surface without leaving a film behind.”
“This discovery was born out of a deep understanding of the science concepts coupled with simple scientific elegance,” says Santamarina. Mishra agrees by concluding that “the strength of our contribution lies in its simplicity.”
References
- Nauruzbayeva, J., Sun, Z., Gallo, A., Ibrahim, M., Santamarina, J.C. & Mishra, H. Electrification at water–hydrophobe interfaces. Nature Communications 11, 5285 (2020).| article
You might also like
Environmental Science and Engineering
Bacteria reveal hidden powers of electricity transfer

Environmental Science and Engineering
Wastewater surveillance tracks spread of antibiotic resistance

Bioscience
Super fungi survive extreme Mars-like environments

Environmental Science and Engineering
Rethinking food systems to restore degraded lands

Environmental Science and Engineering
Combat climate change by eliminating easy targets

Environmental Science and Engineering
Wastewater treatment to fight the spread of antibiotic resistance
Bioscience
Digging into the world of plant-growth-promoting microbes

Bioscience




