Chemistry
Hybrid nanomaterials bristle with potential
Triple-layered nanoarray electrode promises to boost battery performance and enhance other electrochemical processes.
By combining multiple nanomaterials into a single structure, scientists can create hybrid materials that incorporate the best properties of each component and outperform any single substance. A controlled method for making triple-layered hollow nanostructures has now been developed at KAUST. The hybrid structures consist of a conductive organic core sandwiched between layers of electrocatalytically active metals: their potential uses range from better battery electrodes to renewable fuel production.
Although several methods exist to create two-layer materials, making three-layered structures has proven much more difficult, says Peng Wang from the Water Desalination and Reuse Center who co-led the current research with Professor Yu Han, member of the Advanced Membranes and Porous Materials Center at KAUST. The researchers developed a new, dual-template approach, explains Sifei Zhuo, a postdoctoral member of Wang’s team.
The researchers grew their hybrid nanomaterial directly on carbon paper—a mat of electrically conductive carbon fibers. They first produced a bristling forest of nickel cobalt hydroxyl carbonate (NiCoHC) nanowires onto the surface of each carbon fiber (image 1). Each tiny inorganic bristle was coated with an organic layer called hydrogen substituted graphdiyne (HsGDY) (image 2).
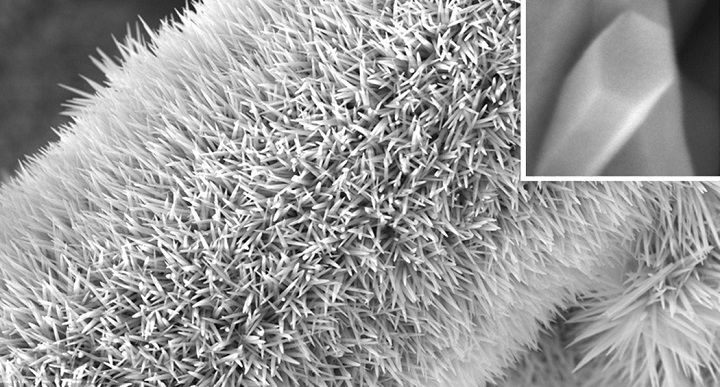
Image 1: A carbon fiber covered with a spiky forest of NiCoHC nanowires.
All images reproduced from reference 1 under a Creative Commons Attribution 4.0 International License© 2018 KAUST
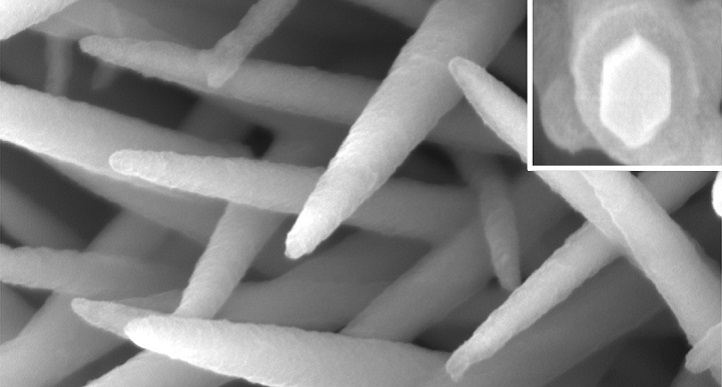
Image 2: Close ups of the nanowires confirm they have been coated with a thin organic layer.
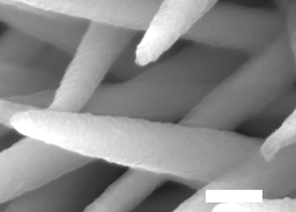
Next was the key dual-template step. When the team added a chemical mixture that reacts with the inner NiCoHC, the HsGDY acted as a partial barrier. Some nickel and cobalt ions from the inner layer diffused outward, where they reacted with thiomolybdate from the surrounding solution to form the outer nickel-, cobalt-co-doped MoS2 (Ni,Co-MoS2) layer. Meanwhile, some sulfur ions from the added chemicals diffused inwards to react with the remaining nickel and cobalt. The resulting substance (image 3) had the structure Co9S8, Ni3S2@HsGDY@Ni,Co-MoS2, in which the conductive organic HsGDY layer is sandwiched between two inorganic layers (image 4).
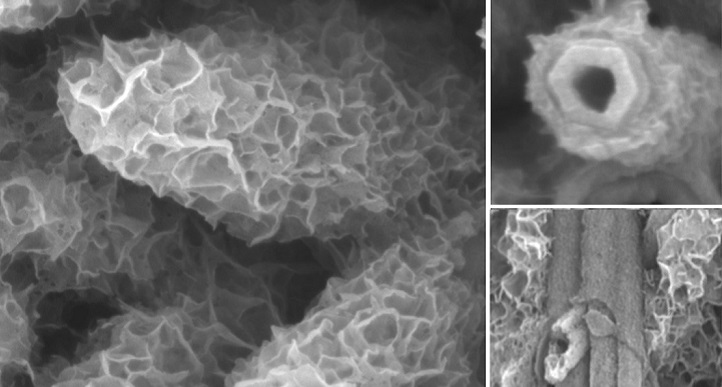
Image 3: The completed triple layer nanowires .
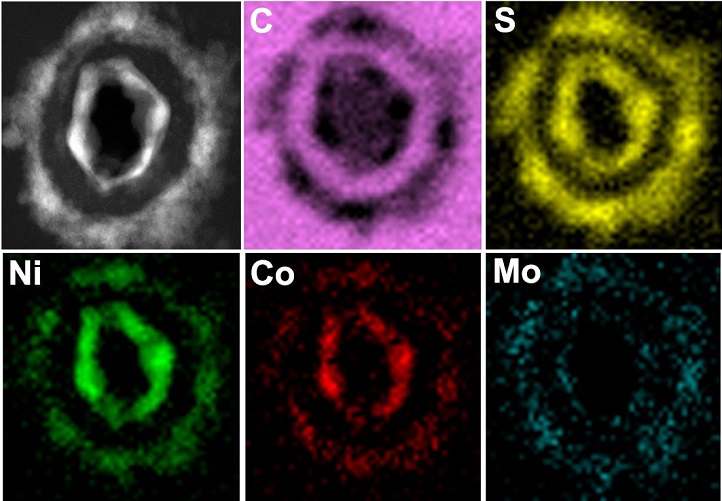
Image 4: Elemental mapping confirmed sulfur (yellow), nickel (green) cobalt (red) and molybdenum (blue) formed the inner and outer layers, sandwiching an organic layer rich in carbon (pink).
The triple layer material showed good performance at electrocatalytically breaking up water molecules to generate hydrogen, a potential renewable fuel. The researchers also created other triple-layer materials using the dual-template approach
“These triple-layered nanostructures hold great potential in energy conversion and storage,” says Zhuo. “We believe it could be extended to serve as a promising electrode in many electrochemical applications, such as in supercapacitors and sodium-/lithium-ion batteries, and for use in water desalination.”
References
-
Zhuo, S., Shi, Y., Liu, L., Li, R., Shi, L. Anjum, D. H. Han, Y. & Wang, P. Dual-template engineering of triple-layered nanoarray electrode of metal chalcogenides sandwiched with H-substituted graphdiyne. Nature Communications 9, 3132 (2018). | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering




