Chemistry
Self-assembled nanostructures hit their target
A biocompatible nanomaterial that can be controlled with light finds a use in gene delivery.
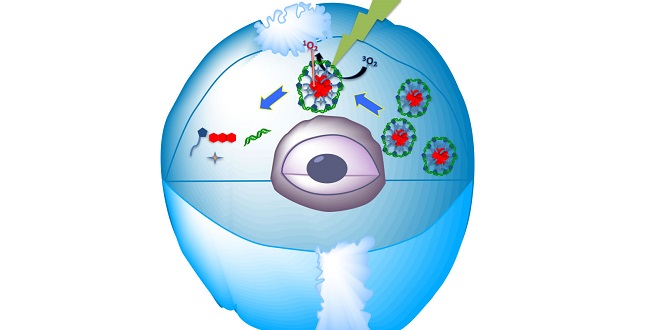
The self-assembled biocompatible nanomaterial delivers the siRNA into the cell and then releases it when struck by light.
© 2016 KAUST
A tiny therapeutic delivery system that can control the body’s ability to manufacture proteins has been developed by KAUST researchers1.
Genes contain the instructions for manufacturing the proteins that make up our bodies. Genetic information is translated into the proteins needed to build living cells through a transcription process in which DNA’s genetic code is copied into a large molecule known as messenger RNA (mRNA).
This transcription process can be altered by introducing short double-strands of RNA called small interfering RNA (siRNA), which bind to the mRNA and inhibit the expression of particular genes. Harnessing this RNA interference for therapeutic applications is difficult and requires a material that can protect the siRNA as it travels through the bloodstream, helping it to penetrate the cell’s outer membrane and deliver it to its target location.
“Delivery of RNA is very tricky as it can be readily digested by cells. Better vehicles are needed so more RNA can be delivered in order to edit genes,” said Niveen Khashab from the KAUST Smart Hybrid Materials Laboratory.
Khashab and her colleagues have now demonstrated biocompatible nanostructures for delivering siRNA and efficiently silencing genes1. They combined the macromolecule histidine-capped-9,10-dialkoxy-anthracene (HDA) and siRNA in water and observed the self-assembly of spherical nanoparticles when the water was slightly acidic but not when it was pH neutral.
Khashab explained that the nanospheres are created by the electrostatic interaction between the positively charged HDA and negatively charged RNA. The two long arms of the HDA supramolecule then wrapped around the siRNA to protect it.
“Our organic linker is able to interact with genetic materials by hydrogen bonds and form a delivery vehicle,” Khashab said. “The approach is scalable and creates reproducible amounts of encapsulated RNA, and it is also biocompatible and safe.”
The nanoparticles could also be activated with visible light. When irradiated by green radiation while in the presence of an acidic fluorescent compound known as eosin, the sphere disassembled and released the siRNA.
The team showed the effectiveness of the nanoparticle for drug delivery on B-cell lymphoma 2, an mRNA molecule that creates proteins for regulating cell death. They showed that their nanostructures enhance the gene silencing efficacy and led to gene knockdown of more than 90 percent after exposure to visible light.
“The next step is to tweak the design to deliver other cargo molecules such as proteins and improve the light response to a higher wavelength in the near infrared,” noted Khashab.
References
-
Patil, S. P., Moosa, B. A., Alsaiari, S., Alamoudi, K., Alshamsan, A. et al. Supramolecular self-assembly of histidine-capped-dialkoxy-anthracene: A visible-light-triggered platform for facile siRNA delivery. Chemistry – A European Journal 22, 1521-3765 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



