Chemistry
Good vibrations for organic semiconductors
Standfirst: Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
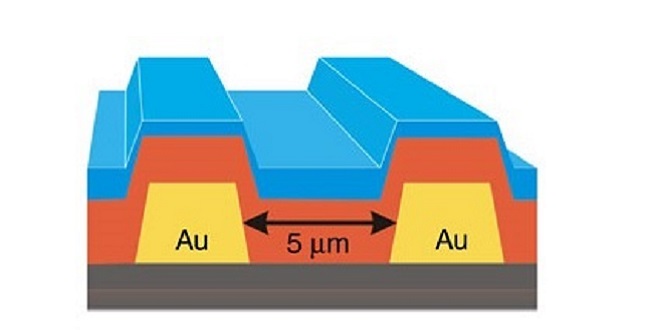
The photoresistor contains thin layers of pentacene (red) and buckminsterfullerene (C60, blue) on top of a gold electrode (Au, yellow).
Reproduced with permission from Ref 1© 2015 Nature Communications/ Bakulin et al.
Organic molecular thin films are increasingly used as semiconductive materials in electronic devices because they are flexible, usually inexpensive and relatively easy to coat surfaces. Their conductivity is extremely sensitive to small changes in the molecule’s shape.
Artem Bakulin at the Foundation for Fundamental Research on Matter (FOM) in the Netherlands and colleagues at KAUST, including Jean-Luc Bredas from the University’s Solar & Photovoltaics Engineering Research Center, have developed a technique to tune the conductivity of organic molecular films by making the molecules vibrate in specific ways1. This strategy could eventually be used to develop organic semiconductors that allow current to flow through them very quickly or to switch organic electronic devices very rapidly.
The researchers tested the method on a photoresistor, which allows more electricity to pass through it when exposed to light. They made the device by coating a gold electrode with a layer of pentacene, a molecule of five benzene rings fused in row. They then added a second layer made from hollow balls of carbon atoms called buckminsterfullerene (C60).
When a pulse of visible light hits the device, it excites the pentacene molecules and leads to electrons being transferred to the C60 molecules at the interface with the C60 layer, leaving positively-charged “holes” behind in the pentacene layer. These holes can then flow through the pentacene film and generate a current.
However, some of the holes get trapped in defects within the pentacene film before they reach the gold electrode. The team then used a pulse of infrared light nearly 1 millisecond after the initial visible pulse to generate vibrations in pentacene molecules to help to release some of these trapped holes.
When a second pulse of visible light follows just 30 trillionths of a second later, these untrapped holes add to the photocurrent, which can thereby increase by more than 10 percent.
“I am not aware of any earlier study that was able to reveal experimentally the impact of specific vibrational modes on the current flowing in organic semiconductors,” Bredas says.
Specific high frequencies of infrared light boosted the current more because they made the pentacene vibrate along the length of the molecule rather than across its width. The theoretical calculations conducted in the Bredas group suggested that lengthwise vibrations helped charge carriers to “untrap” and hop from one molecule to the next.
Aside from enabling more effective organic semiconductor devices, Bredas notes that the work could have more fundamental implications.
“The application of this technique to other organic semiconductors will allow us to better understand the charge-transport mechanism in organic thin films, which is a significant part of our research,” he states.
References
-
Bakulin, A. A., Lovrincic, R., Yu, X., Selig, O., Bakker H. J. et al. Mode selective vibrational modulation of charge transport in organic electronic devices. Nature Communications 6, 7880 (2015).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



