Chemistry
Capturing carbon dioxide
Chemically fine-tuning the structure of a class of light porous materials allows efficient and selective removal of CO2 from air.
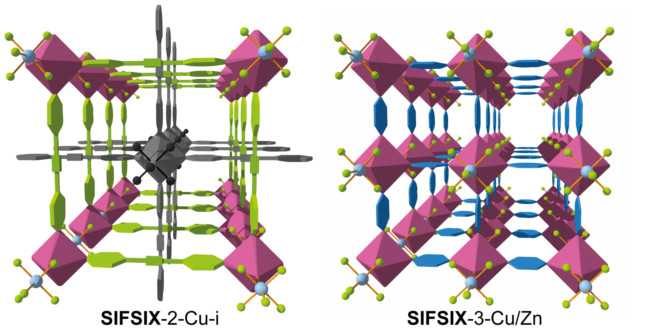
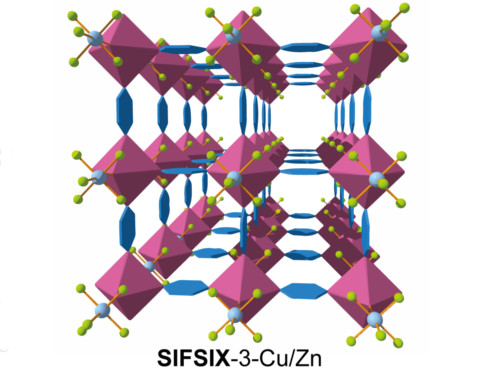
Incorporating pyrazine molecules (blue hexagons) into the SIFSIX structure composed of copper (purple polyhedra), silicon (light blue spheres) and fluorine atoms (light green spheres) creates a porous structure with exceptional selective CO2 adsorption.
© KAUST
As the concentration of carbon dioxide (CO2) in the atmosphere continues to climb, its extraction from air could be made easier and consume less energy through use of a highly selective adsorbent material developed by KAUST researchers1.
“The ever-increasing CO2 in the atmosphere is becoming critical,” says Mohamed Eddaoudi of KAUST’s Advanced Membranes and Porous Materials Research Center. Slowing or reversing this trend is a prominent environmental and political issue that confronts both scientists and policy-makers.
In a bid to find materials that can sequester CO2, Eddaoudi and colleagues drew on their previous success with a diverse groups of porous compounds known as metal-organic frameworks (MOFs) that have an enormous internal surface area and are able to selectively adsorb and store specific gases. MOFs comprise a metal ion (or cluster) and a joining organic molecule known as a linker. The choice of, and ability to alter, these two components confers the MOF’s broad use for different applications including gas storage.
By tweaking the organic and inorganic components, Eddaoudi’s team was able to control the pore sizes of the MOFs which was then able to adsorb CO2 with unprecedented selectivity and efficiency
The CO2-capturing MOF is built around a complex of copper, silicon and fluorine: with its name ‘SIFSIX-3-Cu’ representing the ratios of elements involved — six fluorine atoms per silicon atom.
The organic component of the MOF is called the ‘pyrazine moiety’ which is comprised of a ring of four carbon and two nitrogen atoms with four hydrogen atoms attached. The pyrazine is incorporated into a lattice of copper, silicon and fluorine to produce the crystalline porous and adsorbent structure (see image). This particular material emerged from a wide exploration of different organic components and different metals in the presence of SIFSIX pillars.
The new crystalline powdery MOF is able to adsorb CO2 from dilute gas streams with very low levels of CO2, making it more versatile and efficient than existing alternatives. The researchers suggest that the physical sorption occurs through electrostatic attraction between CO2 molecules and fluorine atoms present on the surface of the adsorbent.
“The next step is to scale up this class of materials and to test them at the pilot plant,” Eddaoudi adds.
This MOF could be used to reduce industrial CO2 emissions from two key sources of carbon pollution, stationary power plants and transportation. It also has application for processes that require a supply of CO2-free air, such as in alkaline fuel cells or breathing systems in submarines or space shuttles.
The researchers also note that these findings contribute to the broader understanding of MOFs — the hotly researched ‘miracle materials’ — as the synthesis methods demonstrate their potential for other gas adsorption and storage applications.
References
-
Shekhah, O., Belmabkhout, Y., Guillerm, V., Cairns, A., Adil, K & Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nature Communications, 5, 4228 (2014). | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



