Chemistry
Nanoparticles take a bite out of infections
Spiky-shaped additives help antimicrobial coatings sense and inhibit bacteria growth on dental devices.
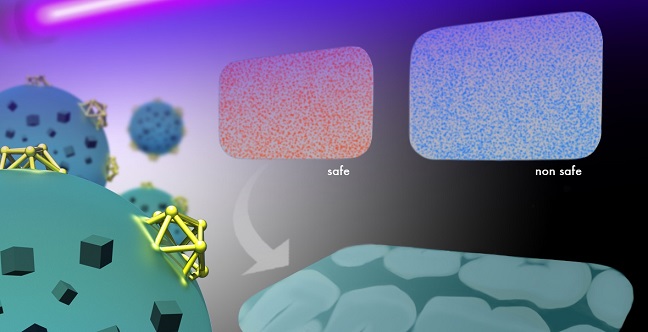
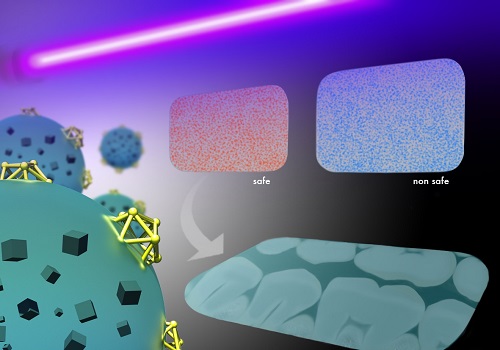
A rapid test under UV light reveals if dental imaging plates are contaminated with bacteria, thanks to polymers embedded with multi-functional nanoparticles.
Reproduced with permission from ref 1.© 2016 Wiley-VCH and KAUST Ivan Gromicho
Antibiotic-resistant bacteria that colonize surfaces and medical equipment are causing alarming annual rises in the number of patients becoming infected in hospitals and clinics. A KAUST team is working to reduce these numbers with a smart polymer that changes color and activates natural antimicrobial enzymes when bacterial contamination is detected.
Constant exposure to salivary bacteria makes dental tools, such as reusable X-ray imaging plates, ideal environments for virulent biofilms. One solution to this problem is to coat devices with polymers embedded with nanoscale crystals that slowly release silver ions, a broad-spectrum biocide agent. However, challenges with nanoparticle leaching, have thwarted advancement of this technology.
Associate Professor Niveen Khashab, her PhD student Shahad Alsaiari and colleagues from the University’s Advanced Membranes and Porous Materials Center realized that switching to gold nanoparticles could give antimicrobial coatings detection capabilities—these tiny crystals have sensitive optical properties that can be tuned to spot specific biomolecular interactions. But incorporating them safely into polymers required new types of nanofillers.
“Nanofillers are small chemical agents distributed in the matrix of a polymer composite,” explains Khashab. “They’re dopants, so they improve on the regular material and introduce new properties—in our case, making the coating antibacterial.”
The team’s approach uses gold nanoclusters treated with lysozyme enzymes that have innate defenses against pathogens, such as Escherichia coli, commonly known as E. coli. They attached these colloids to the surface of slightly larger, porous silica nanoparticles stuffed with antibiotic drug molecules.
Normally, this gold-silica complex emits glowing, red fluorescent light. But when the lysozyme units encounter bacteria, a strong attraction for cell walls rips the gold nanoclusters from their silica partners—an action that simultaneously switches off fluorescence and releases the antibiotic cargo.
Blending experiments revealed the gold-based nanofillers integrated thoroughly into polymer composites and exhibited minimal leaching during trials with E. coli. Khashab attributes these favorable polymer interactions to the sharp exposed edges of gold clusters on the silica spheres
The researchers tested their concept by comparing X-ray dental plates with and without the smart polymer coating. Both samples yielded the same high-resolution images of teeth and bone structure. However, only the coated plate enabled rapid visual assessment of bacterial contamination, simply by illuminating the device with a UV-lamp and looking for color change. Successful release of the antibacterial agent also drastically decreased biofilm buildup.
“The process of coating is easy,” nots Khashab. “We are looking at improving this technology to include other medical devices of different sizes and shapes.”
References
- Alsaiari, S.K., Hammami, M.A., Croissant, J.G., Omar, H.W., Neelakanda, P. ... & Khashab, N.M. Colloidal gold nanoclusters spiked silica fillers in mixed matrix coatings: Simultaneous detection and inhibition of healthcare-associated infections. Advanced Healthcare Materials 5, 1617–1626 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



