Chemistry
Gold standards for nanoparticles
Understanding how small organic ions stabilize gold nanoparticles may allow for better control.
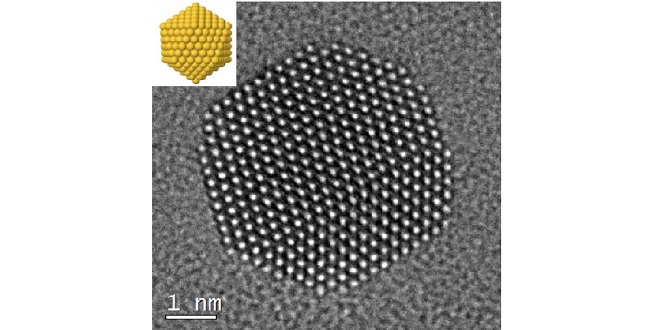
Expanding the potential of gold nanoparticles for a range of uses requires methods to stabilize the clusters and control their size. Researchers at KAUST reveal how simple organic citrate ions, derived from readily available citric acid, can interact with the gold atoms to yield the stable nanoparticles needed for further research.
Such clusters of gold atoms are proving increasingly useful as catalysts, drug delivery systems, anti-cancer agents and components of solar cells among other applications.
“The potential applications of gold nanoparticles could have a huge impact on society, and understanding stabilizers like citrate might be crucial to progress,” said Jean-Marie Basset, Director of the KAUST Catalysis Center and Distinguished Professor of Chemical Science, who cosupervised Hind Abdullah Al-Johani with Professor Luigi Cavallo. With this work, Al-Johani, a recent graduate of KAUST and now Associate Professor of Chemistry at Tabuk University, became the first Saudi woman to be published as a first author in Nature Chemistry.
Along with colleagues at The University’s Core Labs and coworkers in UK, Switzerland and France, the researchers have shown different ways that citrate ions can bind to gold atoms at the surface of nanoparticles1. They also discovered how to influence the binding mode by controlling the ratio of the nanoparticle/citrate ions. Different modes can influence the structures and properties of nanoparticles.
“The experimental and theoretical characterization of these systems is challenging due to the flexible nature of the interaction between citrate and gold,” said Basset. He explained that collaboration between KAUST teams was essential to meet the challenges of creating stabilized nanoparticles and analyzing and imaging them at high resolution (see image).
One reason for gold’s usefulness in medical applications is its chemically stable nature. Other researchers have shown that this stability allows gold to carry drugs through the body without causing chemical side effects.
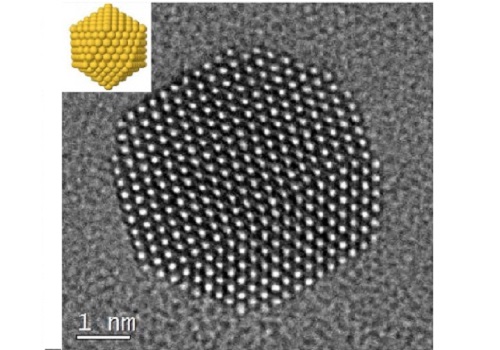
Gold nanoparticles imaged at atomic resolution with an idealized schematic at top left.
© Reproduced with permission from ref 1. © 2017 Nature Publishing Group
Controlling the structure of gold nanoparticles could also fine tune their interaction with light to exploit a phenomenon known as surface plasmon resonance. This may allow the energy of light to be harnessed to kill cancer cells. Attaching antibodies can guide the nanoparticles to the specific cells that need treatment. The type of interaction with light depends on nanoparticle structure and could also yield applications in solar cells and microelectronics.
The researchers consider that the insights from this work at KAUST may also be applicable to some other metals and plan to explore this as the next phase of the research. “We want to take on that wider challenge,” said Basset.
References
-
Al-Johani, H., Abou-Hamad, E., Jedidi, A., Widdiefield, C. M., Viger-Gravel, J. …& Basset, J.-M. The structure and binding mode of citrate in the stabilization of gold nanoparticles. Nature Chemistry 9, 890-895 (2017).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



