Chemistry
Making use of flat surfaces for catalysis
Using a plasma to alter the atomic composition of two-dimensional materials could make them better catalysts.
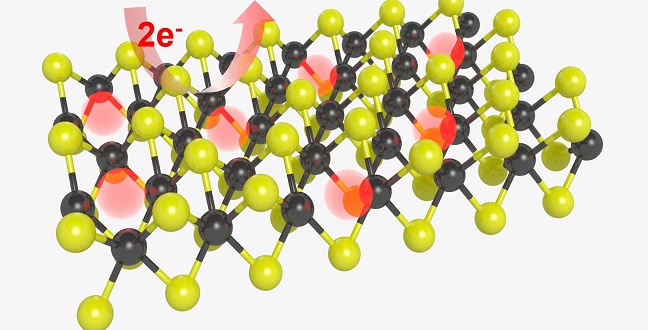
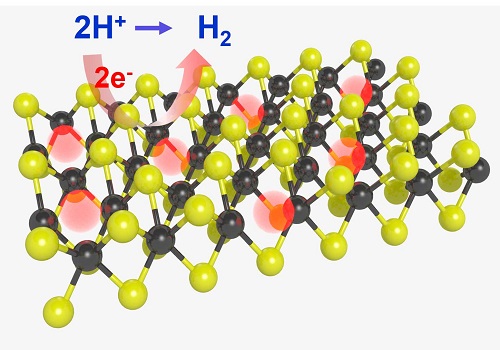
Treating molybdenum disulfide in a hydrogen plasma creates active sites on its planar surface and makes the surface reactive for reducing water to hydrogen.
© 2017 KAUST
A novel way of increasing the chemical reactivity of a two-dimensional material has been used to produce a cheap and effective catalyst that can extract hydrogen from water—an important step in the quest for clean energy supplies. This technique developed by KAUST researchers may also have potential benefits for other manufacturing industries1.
Hydrogen stores an enormous amount of energy that can be released by electrochemically combining hydrogen and oxygen in a fuel cell. Despite being the lightest element in the periodic table, gaseous hydrogen doesn’t occur naturally on Earth, so the race is on to find an efficient process to produce it.
One route to hydrogen generation is by electrolysis: passing an electrical current through water via two electrodes to cause a chemical reaction that breaks the water molecule into its component hydrogen and oxygen atoms. The speed of this so-called hydrogen evolution reaction can be increased using a catalyst on the electrodes.
Platinum is a perfect material for the job, but is it very expensive.
Lain-Jong Li, Professor of Material Science and Engineering at KAUST, with colleagues from the National Chiao Tung University and the National Applied Research Laboratories in Taiwan have demonstrated a method for increasing the catalytic activity of molybdenum disulfide, a much cheaper alternative.
Molybdenum disulfide is a two-dimensional material very similar to graphene. Previous experimental and theoretical results have verified its excellent catalytic potential and indicated that the hydrogen evolution reaction takes place at its jagged edges, while its flat surface planes remain chemically inert.
“A monolayer of molybdenum disulfide is only reactive for reducing water to hydrogen at its edge,” explains Li. “But we discovered an efficient way to create active sites on its planar surface, largely activating surface reactivity.”
Li and team created their molybdenum disulfide using a process called chemical vapor deposition. A sample was then transferred to a graphite substrate and placed in a vacuum chamber in which the researchers created a hydrogen plasma. This process removed some of the sulfur atoms from the surface of the sample. By adjusting the sample’s time in the plasma, the team could control the density of these sulfur vacancies.
The researchers confirmed the changes in catalytic activity and also found a useful offshoot of this process. Controlling the atomic composition of molybdenum disulfide could also lead to the development of electrical, optical and magnetic devices.
“Next we hope to move beyond the beaker and put the catalysts in practical flow cell tests,” says Li.
References
- Cheng, C.-C., Lu, A.-Y., Tseng, C.-C., Yang, X., Hedhili, M. N., Chen, M.-C., Wei, K.-H. & Li, L.-J. Activating basal-plane catalytic activity of two-dimensional MoS2 monolayer with remote hydrogen plasma. Nano Energy 30, 846–852 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



