Chemistry
A simple nose for noxious gases
Tunable porous MOF materials interface with electrodes to sound the alarm at the first sniff of hydrogen sulfide.
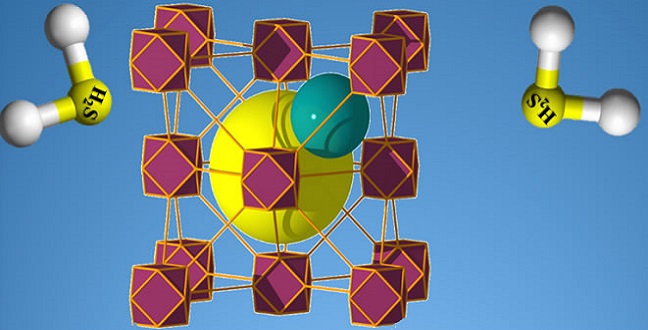
A carefully tuned MOF can detect the presence of hydrogen sulfide gas with ppb sensitivity.
© 2017 KAUST
A thin-film chemical sensor coated onto an electrode offers a simple, practical way to detect minute traces of toxic gas. Sensors that use metal-organic frameworks (MOFs) can be highly selective for a particular gas because of the porous nature of these crystalline materials and their nanoscale cavities. Recently, KAUST researchers developed a MOF-based sensor that can selectively sense hydrogen sulfide at concentrations of just a few parts per billion (ppb). Their method of altering the structure of the MOF-electrode coating allows them to detect a range of gases.
Better known as “sewer gas” for its characteristic rotten-egg odor, hydrogen sulfide doesn’t just smell bad; it is harmful to humans at concentrations as low as just a few parts per million (ppm). In industrial settings, where hydrogen sulfide release is a risk factor, including oil and gas refining and sewage treatment plants, gas-sensing systems based on gas chromatography are typically deployed; however, these systems are bulky, complex and expensive.
A much simpler sensor has now been developed through collaboration between the KAUST research groups of Khaled Salama, professor of electrical engineering, and Mohamed Eddaoudi, professor of chemical science. By altering the metal and organic components of the MOFs used in Eddaoudi’s sensors, the team fine tuned the size and shape of the pores and altered the cavities where gases enter. In this way, the material becomes highly selective at adsorbing a particular gas.
Eddaoudi recently developed MOFs based on rare earth metals using particular organic linkers that allowed the construction of a MOF with a face-centered cubic (fcu) topology. He explains that this topology is showing a promising selectivity for the adsorption of hydrogen sulfide. “The exceptional thermal and chemical stability of these MOFs and their high selectivity for the adsorption of H2S encouraged us to explore the fcu-MOF platform for the sensing of toxic gases, such as H2S.”
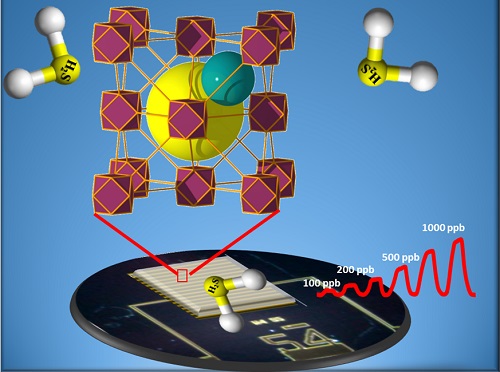
To turn the MOF into a sensor, the team grew a thin coating of the fcu-MOF on an electrode. When hydrogen sulfide enters the MOF, it alters an electromagnetic property of the coated electrode called relative permittivity, which is how the sensor detects the presence of the gas.
“Unexpectedly, the sensor was not only able to perform well in the detection of H2S gas in the ppm range but also in the ppb range with a detection limit of about 5.4 ppb,” Eddaoudi says. “This exceptional sensing performance paves the way for the deployment of MOFs as practical sensors for various detection applications of toxic gases and vapors.”
References
- Yassine, O., Shekhah, O., Assen, A.H., Belmabkhout, Y., Salama, K.N. & Eddaoudi, M. H2S sensors: fumarate-based fcu-MOF thin film grown on a capacitive interdigitated electrode. Angewandte Chemie International Edition 55, 15879 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



