Chemistry
Solid progress in carbon capture
New heights reached for solids that capture carbon dioxide at low concentrations in gas mixtures.
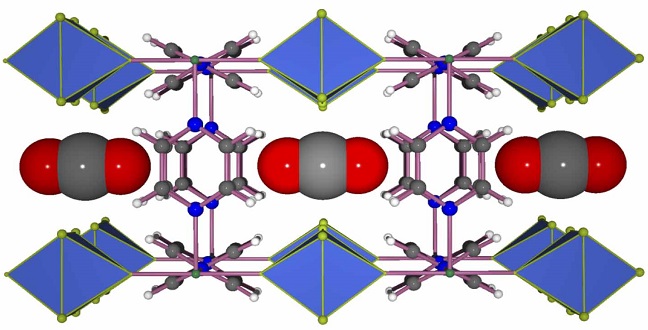
Carbon dioxide capture is a high-profile area of chemical research offering a direct approach to tackling the rise in atmospheric carbon dioxide. This greenhouse gas is largely blamed for global warming and climate change.
Mohamed Eddaoudi, Associate Director of the University’s Advanced Membranes and Porous Materials Research Center, leads a team of researchers at KAUST who are developing porous solids called metal-organic frameworks (MOFs) for the selective removal of various gases from gas mixtures. Their latest breakthrough material can effectively take up carbon dioxide even when it is present at concentrations as low as 400 parts per million and opens possibilities for capturing CO2 as it is generated.
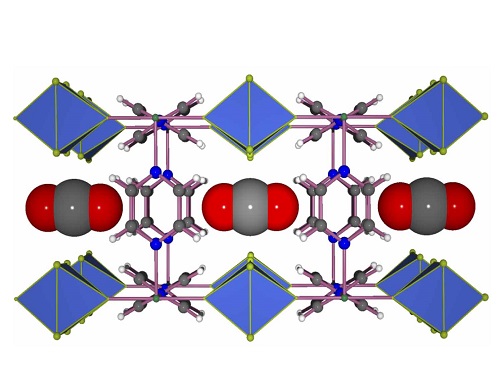
Carbon dioxide molecules (gray and red) fit neatly into the channels of the MOF.
© 2016 KAUST
MOFs contain metal ions or clusters that are held in place by organic molecules known as linkers. Altering the chemical composition and geometry of these two primary components can produce versions with varying and highly selective abilities to adsorb and store gases.
“The discovery of this latest material for capturing carbon dioxide is the result of about four to five years of work on this unique MOF platform,” said Eddaoudi. He explained that the key challenge was to create something that could exceed the performance of existing options while also greatly reducing the energy requirements over the full cycle of operation.
The researchers’ response was to develop a fluorine-containing MOF in which square-grid layers encompassing Ni(II) metal centers and pyrazine linkers are bridged via pillars composed of niobium, oxygen and the fluorine atoms1.
“The ability to control the distance between the fluorine atoms allowed us to create the ideal square-shaped pockets for trapping carbon dioxide molecules effectively and efficiently and giving our material such impressive performance,” said Eddaoudi.
The location of carbon dioxide molecules inside the MOFs was visualized using X-ray diffraction equipment at the University of Stellenbosch in South Africa.
The ability to trap carbon dioxide when it is at very low concentrations makes the new material suitable for a wide range of applications, including the direct capture from air.
Eddaoudi explained that the MOF might be adapted for use in static industrial processes that generate carbon dioxide (such as cement factories), but could also be used on board vehicles such as trucks, cars and aircraft. Capturing the carbon dioxide as soon as it is emitted could be significantly more effective and efficient than going after it when it has mixed in with the atmosphere overall.
“We are now working to scale up the use of this material, allowing us to seek industry collaboration towards eventual commercialization,” Eddaoudi said.
References
-
Bhatt, P.M., Belmabkhout, Y., Cadiau, A., Adil, K., Shekhah, O., Shkurenko, A., Barbour, L.J. & Eddaoudi, M. A fine-tuned fluorinated MOF addresses the needs for trace CO2 removal and air capture using physiosorption. Journal of the American Chemical Society 138, 9301 – 9307 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



