Chemistry
A clean separation
The efficient purification of propylene using a fine-tuned porous material delivers advances in petrochemical production.
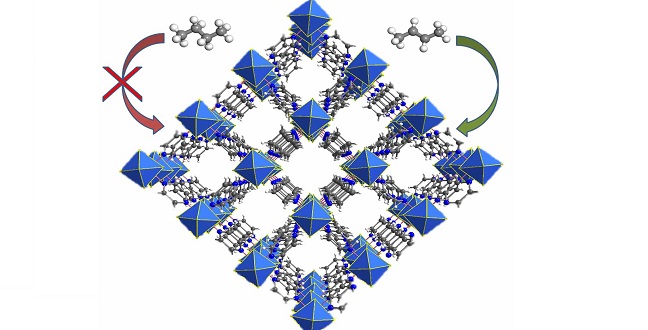
KAUST-7, a metal-organic framework (MOF) (blue) that adsorbs propylene (green) and rejects propane (red).
© 2016 KAUST
A breakthrough for the production of a key component for popular polymer materials has been achieved by researchers from KAUST. Mohamed Eddaoudi and colleagues have developed a porous material that easily removes propane, a detrimental gas, from propylene, improving the potential for efficient fabrication of polypropylene polymers1.
“Unveiling this unique material and its distinctive separation properties was a real tour de force in metal organic framework [MOF] chemistry and materials design,” said Eddaoudi.
Polypropylene based polymers can be shaped into many forms and are widely used in the multibillion dollar industry to produce plastic goods like food containers or furniture. The base compound, propylene, needs to be at least 99.5 percent pure, and present-day purification techniques based on a cryogenic distillation process are expensive and energy-intensive.
The approach discovered by the researchers employs preferential adsorption, in which one compound is reversibly captured by a porous material and the other is rejected. Ideally suited for this task are MOFs, which are porous, three dimensional structures in which inorganic building blocks are linked by organic molecules.
The MOFs are large enough to capture a variety of gases depending on the size of the pore system. A key factor in determining whether a molecule is adsorbed is the pore aperture size. Larger apertures are not efficient in differentiating adsorption of molecules. Therefore, it is important that the entrance to the pores is fine-tuned on the atomic scale to the dimensions of the target molecules.
In previous work, the researchers developed a class of MOFs suitable for processes such as carbon dioxide capture, but these were unsuitable to separate propane from propylene, as these two molecules are very similar in shape and size.
The researchers have now replaced the silicon-based inorganic building blocks of that MOF with ones based on niobium, which are slightly larger. The overall crystal structure of the MOF remains the same, leading to a reduced pore aperture size of the new MOF.
This compound, which is named KAUST-7, achieves an efficient separation between propane and propylene at room temperature and ambient pressures. The separation of the two molecules retains its efficiency for at least eleven repeated cycles and works robustly, noted Eddaoudi.
“KAUST-7 is unique not only because it displays an impressive performance better than current benchmark materials, but also because it maintains distinctive separation properties in the presence of water as a result of its high chemical stability,” he added.
References
-
Cadiau, A., Adil, K., Bhatt, P.M., Belmabkhout, Y. & Eddaoudi, M. A metal-organic framework based-splitter for separating propylene from propane. Science 353, 137-140 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



