Chemistry
Building blocks for methane gas storage
Tweaking the structure of metal-organic frameworks could transform the capacity to use methane as a fuel.
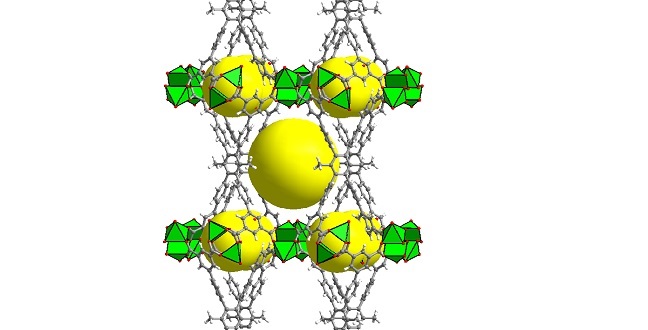
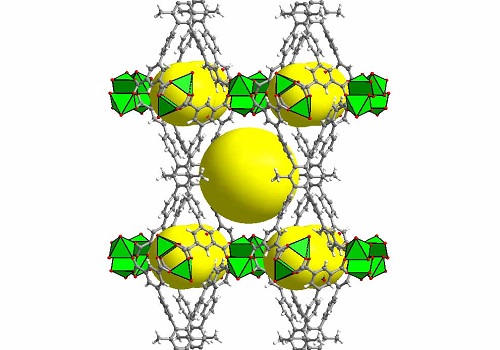
Geometric structure of the methane-storage MOF. Organic linkers (gray/red) and metallic centers (green) combine to create cavities (yellow) that can store many molecules of methane.
Reproduced with permission from reference 1© 2016 American Chemical Society.
A hybrid material that could lead to cheaper and more effective methane storage has been created by a globally prominent research team at KAUST, with collaborators at the University of Crete.
Natural gas, which is almost 95 percent methane, is a good candidate for replacing gasoline and coal. It can provide the same amount of energy as these fossil fuels, while releasing much less of the greenhouse gas carbon dioxide and the toxic pollutants carbon monoxide, nitrogen oxides and sulfur oxides. Methane is more environmentally friendly in several ways, but its widespread adoption for powering vehicles and other local and mobile applications is limited by shortcomings of existing storage and transport technologies.
Mohamed Eddaoudi of KAUST’s Advanced Membranes and Porous Materials Research Center leads a wide range of research projects involving metal-organic frameworks, or MOFs. These hybrid materials contain single metal ions or metal clusters held together by carbon-based ‘organic’ chemical groups known as linkers. Rearranging different linker and inorganic molecular building blocks allows scientists to fine-tune the size and chemical properties of the pore system in MOFs to perform useful functions. These include highly selective gas adsorption and catalysis.
“MOFs are considered by far the best class of materials for storing gases, especially methane,” says Eddaoudi. He explains that tinkering with different pore sizes can create exceptionally large internal surface areas that allow MOFs to hold greater amounts of gas than other porous substances. MOF-making can be likened to using toy building blocks to assemble a wide range of open geometric networks. Diagrams representing these structures actually look like colorful toys (see image) but built at the atomic and molecular scale. The key to the latest MOF is the choice of the appropriate organic ‘pillars’ (shown in gray in the image) used to create two types of cavities that can each contain many of the methane molecules taken up by the MOF.
Further tweaking is required. “We need to boost the methane storage capacity and cyclability of the MOFs, enhance their stability in water, and explore scaling up to commercially useful quantities,” says Eddaoudi. He is confident that the properties of the MOFs can be optimized because of the unique flexibility allowed by the linker and metal structure. Eddaoudi predicts that commercially produced MOFs will be efficiently and effectively storing and transporting methane inside the next decade. Such a development could herald real progress in weaning society off its dependence on oil and coal.
References
- Spanopoulos, I., Tsangarakis, C., Klontzas, E., Tylianakis., E., Froudakis, G. et al. Reticular synthesis of HKUST-like tbo-MOFs with enhanced CH4 storage. Journal of the American Chemical Society 138, 1568-1574 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



