Chemistry
Catching more of the sun
Combining quantum dots and organic molecules can enable solar cells to capture more of the sun’s light.
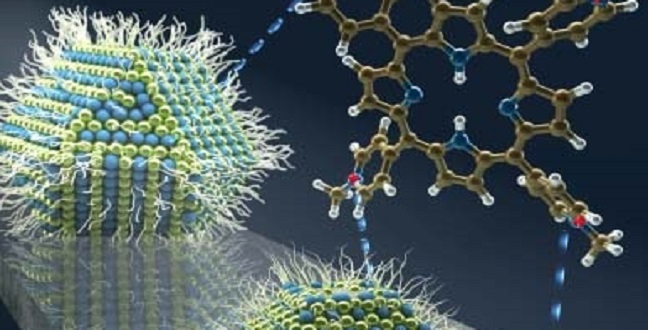
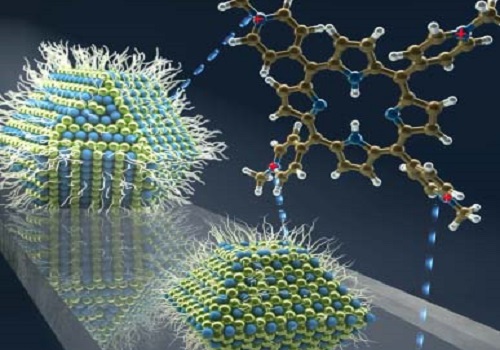
Organic molecules aid charge transfer from large lead sulfide quantum dots for improved solar cell performance.
© 2015 Wiley.
Light from the sun is our most abundant source of renewable energy, and learning how best to harvest this radiation is key for the world’s future power needs. Researchers at KAUST have discovered that the efficiency of solar cells can be boosted by combining inorganic semiconductor nanocrystals with organic molecules.
Quantum dots are crystals that only measure roughly 10 nanometers across. An electron trapped by the dot has quite different properties from those of an electron free to move through a larger material.
“One of the greatest advantages of quantum dots for solar cell technologies is their optical properties’ tunability,” explains Omar Mohammed. “They can be controlled by varying the size of the quantum dot.”
Mohammed and his colleagues are developing lead sulfide quantum dots for optical energy harvesting; these tend to be larger than dots made from other materials. Accordingly, lead sulfide quantum dots can absorb light over a wider range of frequencies. This means they can absorb a greater proportion of the light from the sun when compared to other smaller dots.
To make a fully functioning solar cell, electrons must be able to move away from the quantum dot absorption region and flow toward an electrode. Ironically, the property of large lead sulfide quantum dots that makes them useful for broadband absorption — a smaller electron energy bandgap — also hinders this energy harvesting process. Previously, efficient electron transfer had only been achieved for lead sulfide quantum dots smaller than 4.3 nanometers across, which caused a cut-off in the frequency of light converted.
The innovation by Mohammed and the team was to mix lead sulfide quantum dots of various sizes with molecules from a family known as porphyrins. The researchers showed that by changing the porphyrin used, it is possible to control the charge transfer from large lead sulfide dots; while one molecule switched off charge transfer altogether, another one enabled transfer at a rate faster than 120 femtoseconds.
The team believe this improvement in energy harvesting ability is due to the interfacial electrostatic interactions between the negatively charged quantum dot surface and the positively charged porphyrin.
“With this approach, we can now extend the quantum dot size for efficient charge transfer to include most of the near-infrared spectral region, reaching beyond the previously reported cut-off,” states Mohammed. “We hope next to implement this idea in solar-cells with different architectures to optimize efficiency.”
References
- El-Ballouli, A.O., Alarousu, E., Kirmani, A.R., Amassian, A., Bakr, O.M. & Mohammed O.F. Overcoming the cut-off charge transfer bandgaps at the PbS quantum dot interface. Advanced Functional Materials 25, 7435–7441 (2015).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



