Chemistry
Defying symmetry for better synthesis
Insight into the role of sulfur-bearing ligands provides a better handle on ways to synthesize asymmetric metal nanoparticles.
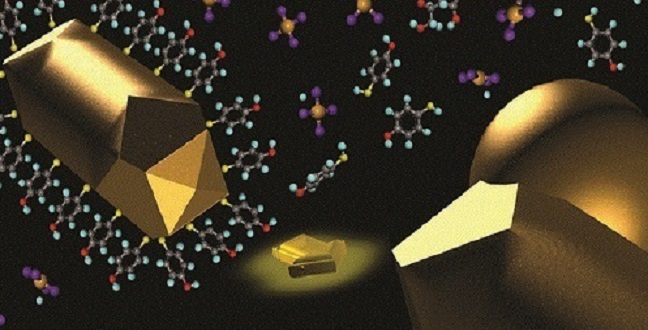
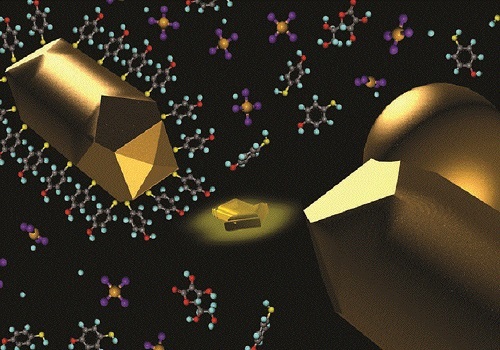
Growth of a gold nanosphere on a thiol-capped gold nanorod acting as a seed.
© 2016 KAUST
Tailored asymmetric nanoparticles are about to become easier to produce following a major breakthrough by KAUST researchers. The researchers studied the growth mechanism to expose the role of thiols, a key type of ligand1.
Asymmetry is expected to give rise to unique electronic and optical properties that are helpful for nanotechnologies such as ultrafast information transfer and sensing. However, asymmetry has shortcomings when it comes to synthesis.
Approaches using particles as seeds have led to bimetallic nanostructures such as gold–silver and palladium–gold crystals. However, asymmetric gold–gold and gold–silver dimers are difficult to obtain because their components present little or no crystal lattice mismatch, a main driving force for dimer formation.
Thiol ligands promote the seeded growth of well-defined asymmetric particles, but to date this contribution has been obscured by the poor quality of the seed nanoparticle crystals.
“This prevents a reliable analysis which is crucial for unraveling the role of thiols,” said team leader Yu Han from the KAUST Advanced Membranes & Porous Materials Center.
To improve the crystal quality, the researchers, in collaboration with Andrea Fratalocchi, KAUST assistant professor of electrical engineering, generated dimers by growing a single gold nanosphere on single-crystalline gold nanorods in the presence of the thiol 4-mercaptophenol.
“This allowed us to identify an interface between dimer components and track the evolution of the dimer structure by transmission electron microscopy,” Han stated.
Han’s team incubated the nanorod seeds with 4-mercaptophenol to replace their original ligand cover before introducing the nanoparticle precursor and the reducing agent ascorbic acid. The resulting nanostructures ranged from peanut-shaped nanorods in the absence of thiols to the desired dimers with increasing thiol concentration. In these unprecedented dimers, the nanorod retained its crystallinity, whereas the nanosphere attached to the nanorod neck via metallic bonding and displayed a twinned crystal structure.
The researchers discovered that the ligands play a dual role in the dimer formation. Thiols modulate the gold precursor reduction rate and aid dimer formation by slowing down this reduction.
“Thermodynamically, they induce significant and inhomogeneous surface strains on the seeds, which distinguishes the most strained site from other symmetry-equivalent sites,” noted Han.
This most strained site produces one stacking fault that breaks the symmetry of the seed and provides a nucleation point for the nanosphere. Subsequent particle growth quickly proceeds at this point through continuous twinning.
The team is currently exploiting this growth mechanism to develop new asymmetric nanoparticle dimers.
“We are planning to use these dimers for light harvesting and photo-thermal conversion,” Han said.
References
- Huang, J. , Zhu, Y., Liu, C., Shi, Z., Fratalocchi, A. et al. Unravelling thiol’s role in directing asymmetric growth of Au nanorod−Au nanoparticle dimers. Nano Letters 16, 617–623 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



