Chemistry
Nano-Lego for clean energy
Novel solid-state materials may hold the key to cleaner fuels and cheap, efficient gas storage.
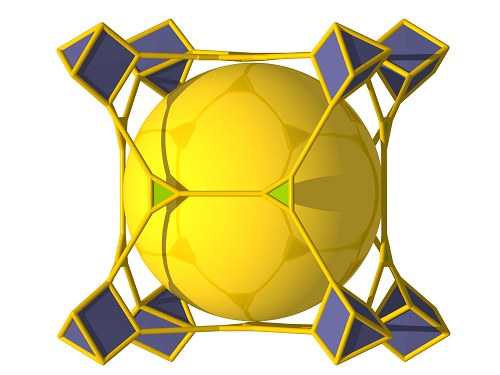
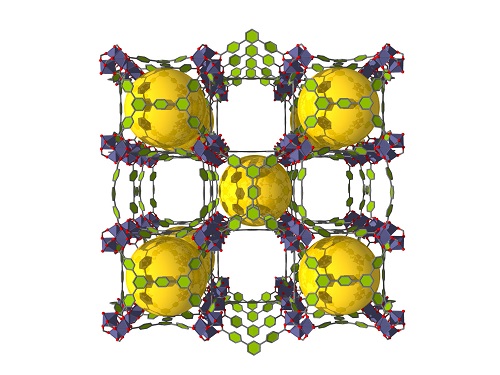
The “Al-soc-MOF” structure comprised a cubic-shaped cage with eight aluminum metal ion clusters (grey) on the vertices (above). These are connected by organic ligands (green) to create a material with cages (yellow) and channels (spaces in between) tailored for specific gas storage (below).
© 2015 KAUST

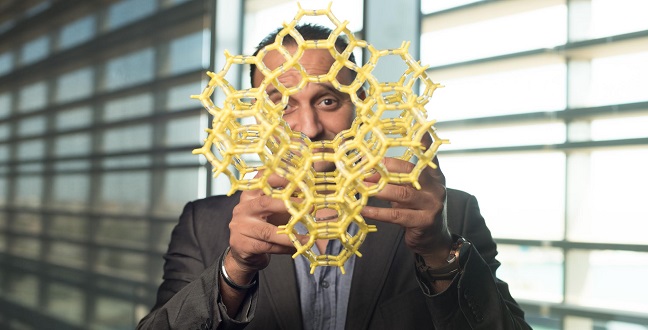
KAUST’s Mohamed Eddaoudi shows off a model MOF at the University.
© 2015 KAUST
Materials developed by researchers at KAUST have the potential to improve the quest for green energy, including the search for cleaner fuels and the means to store various gases.
In recent years, there has been a surge of interest in a group of fabricated materials known as metal-organic frameworks (MOFs). These materials are entirely synthetic and are created from preselected molecular building units into networks of metal ions connected by organic linkers. Their flexible structures can be manipulated for a multitude of highly specific applications.
KAUST is fortunate to have the services of a leading expert in MOFs. Mohamed Eddaoudi from the University’s Physical Science and Engineering Division has devoted his career to designing and building MOFs. The University’s Advanced Membranes and Porous Materials Center, where he conducts his research, was purpose-built to support the KAUST ambition of the University becoming a world-class center for research into sustainable clean energy and power.
“The energy costs associated with the separation and purification of industrial commodities such as gases, fine chemicals and fresh water currently represent around 15 percent of global energy production, and the demand for such commodities is projected to triple by 2050,” Eddaoudi said. “The ongoing challenge is to develop effective separation and purification technologies that have much smaller energy footprints.”
There is also a pressing need to find ways of storing gases—either to remove CO2 from the atmosphere or to enable the storage of cleaner fuels such as hydrogen and methane (CH4) for power. Functional solid-state materials like MOFs could fill this gap in technology.
MOFs are designed from the molecular level up, rather like custom-building with nanoscale Lego bricks. Eddaoudi described how he has developed various design strategies based on the molecular building block approach for the construction of MOFs.
“We select particular metal ions (either individual ions or clusters of two or more together) and then design the organic ligands to bridge the ions/clusters together prior to the MOF fabrication process,” he said. “By doing this, we can control the MOF assembly and determine their nanoscale structure for pre-defined purposes.”
Eddaoudi hopes that improving the processes used to create MOFs will lead to materials being scaled up for use on an industrial scale.
Two recent projects by Eddaoudi and his team highlight the promise of MOFs for gas storage and fuel separation.
The first study, which was led by Eddaoudi’s colleague Youssef Belmabkhout and Ph.D. student Dalal Alezi, used novel aluminum-based MOFs to store CH4, a cheaper and cleaner alternative to existing fuels.
The researchers settled on a “soc-topology” MOF design, a structure based on linking square and octrahedral-shaped building units. The resultant soc-MOF structure encloses channels and cubic-shaped cages with eight metal ion clusters on the vertices of the cage.
The team designed a rectangular organic linker that would help in the automatic formation of clusters of three aluminum ions. The resulting “Al-soc-MOF” showed exceptional porosity, with the highest-ever recorded CH4 uptake at pressures of 35 bar and above, fulfilling the U.S. Department of Energy’s target for CH4 storage.
The same Al-soc-MOFs also showed high storage capacity for CO2 and O2. This suggests that if the MOFs could be scaled up, they could provide solutions for fuel storage, CO2 reduction and improvements to medical devices requiring O2 storage.
“Another of our projects explores how MOFs may help in the separation of light hydrocarbons, one of the most energy-intensive and demanding process in the gas, oil and fuel industries,” said Eddaoudi. “Our team recently succeeded in developing new, cost-effective and energy-efficient adsorbent materials for sieving—and therefore separating—paraffins.”
Branched paraffins are valuable in gasoline production because they create a more efficient fuel. Similarly, normal paraffins are highly prized in diesel fuel. The research team led by Ph.D. student Ayalew Assen under Belmabkhout’s supervision created an MOF based on rare earth metal ions and replaced longer linkers with shorter, dicarboxylate-based organic ligands called fumarate.
The fine-tuning of the size of triangular-shaped apertures in the MOFs allowed for the complete molecular sieving of paraffins. The normal paraffins pass through the holes and the branched paraffins are excluded.
“The MOF materials synthesized at KAUST show great promise to reduce the prominent energy footprint associated with existing technologies,” Eddaoudi noted. “Once we are able to scale-up MOF production, there is no end to the potential of this technology.”
References
- | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



