Chemistry
A greener approach to a green solution
A solvent-free technique for creating adsorbent materials could reduce carbon dioxide emissions.
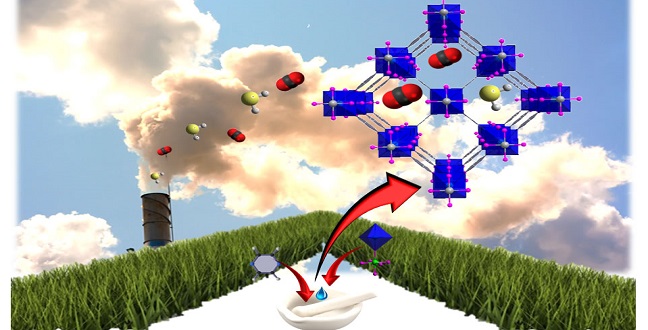
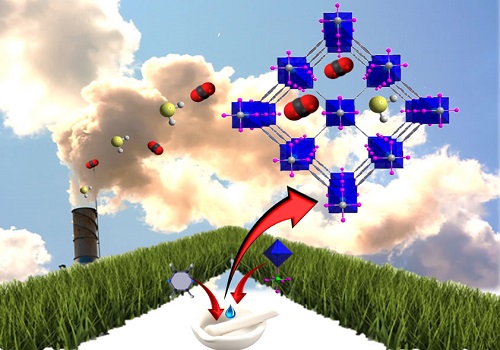
The deliberately tailored nano-space in a metal-organic framework can adsorb and store emitted carbon dioxide.
© 2015 KAUST
An environment-friendly method for synthesizing a microporous material that can adsorb carbon dioxide emitted from fossil fuel-driven power plants has been developed by researchers at KAUST1.
Burning carbon-based energy sources to meet the world’s energy demands is recognized to have a negative impact on our planet: global warming and ocean acidification could leave an indelible mark on Earth. The slow development process and low efficiency of alternatives such as nuclear fusion and solar power makes it difficult to wean ourselves off the use of conventional fossil fuels.
An alternative strategy is to develop technologies that mitigate the deleterious effects of fossil fuels. Carbon capture is one such approach, and proposes to use porous materials that can adsorb and store emitted carbon dioxide at the end of the energy generation process to prevent it from entering the atmosphere.
Metal–organic frameworks (MOFs) are one promising class of porous solid-state materials. These crystalline networks are made up of metal ions or clusters interconnected by organic molecules.
“The periodic arrangement of these organic and inorganic molecular building blocks gives MOFs one of their most defining properties: a functional and tunable pore system,” said KAUST Professor of Chemical Science Mohamed Eddaoudi. “The deliberate control of the available and accessible space shape, size and functionality enables adsorbing and storing select gases.”
The translation of a prospective MOF that selectively captures carbon dioxide from a laboratory scale to industrial scale settings requires the development of economical synthetic approaches. The manufacturing process frequently involves organic solvents that can also have a negative impact on the environment.
Eddaoudi and colleagues from KAUST’s Advanced Membranes & Porous Materials Research Center have developed a simple and solvent-free method to create a MOF adsorbent that selectively captures carbon dioxide.
The reported MOF structure, which they call SIFSIX-3-Ni, was made by dry mechanical mixing the organic component pyrazine with the inorganic solid NiSiF6 at a molar ratio of four to one, and then wetting with a few drops of water. This was heated for four hours at 65 degrees Celsius and then at 105 degrees Celsius for an additional four hours.
The team confirmed the efficient adsorption of carbon dioxide, even in an environment with very low carbon dioxide content. The authors also proved that the material is tolerant to the acidic gas hydrogen sulfide that is present in natural gas.
References
- Shekhah, O., Belmabkhout, Y., Adil, K., Bhatt, P. M., Cairns, A. J. & Eddaoudi, M. A facile solvent-free synthesis route for the assembly of a highly CO2 selective and H2S tolerant NiSIFSIX metal–organic framework. Chemical Communications 51, 13595-13598 (2015). | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
Natural polymer boosts solar cells

Chemistry
Disruptive smart materials flex with real world potential

Chemistry
Catalysts provide the right pathway to green energy

Chemistry
Hollow molecules offer sustainable hydrocarbon separation

Chemistry
Maximizing methane

Chemistry
Beating the dark current for safer X-ray imaging

Chemical Engineering



