Bioengineering
Skeletal scaffold supports bone cells and blood vessels
3D models of bone formation provide a tool for tissue engineering, biomedical research and drug testing.
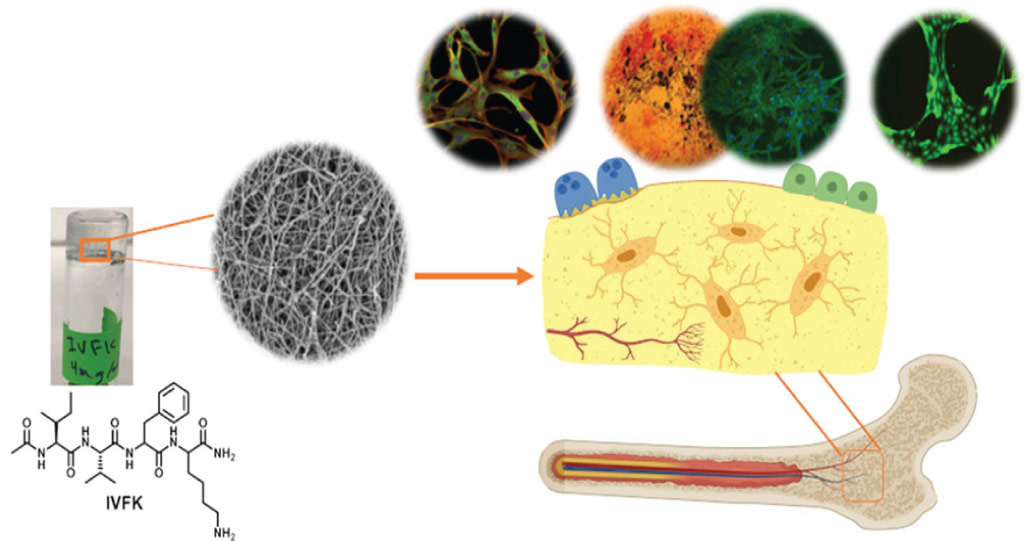
Tissue-engineering scaffolds built around ultrashort peptides provide a new platform for studying bone regeneration in the lab.
The peptides developed at KAUST self-assemble into a cartilage-like hydrogel that mimics the natural matrix that underpins bone formation in the body. Its physiologically relevant properties enable this cell-friendly biomaterial to support the growth and development of bone marrow precursor cells. It also enables tubular blood vessels to take shape, which is a critical part of bone health and repair.
“Our system is a simple, efficient and robust model that closely resembles the complex architecture of native bone tissue,” says Ph.D. student Salwa Alshehri. “Using these peptide-based hydrogels, we can now build 3D disease models for tissue engineering, biomedical research and drug testing.”
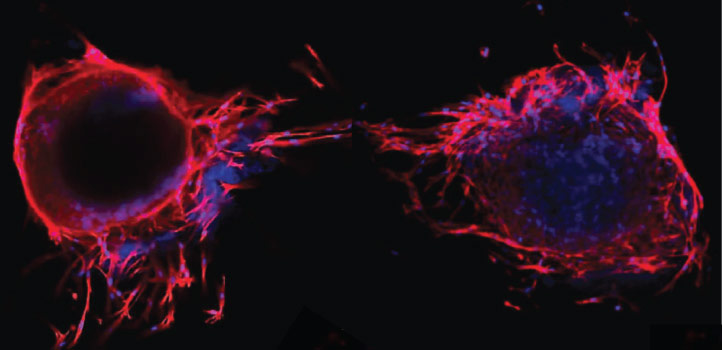
The team seeded their peptide scaffolds with bone marrow–derived mesenchymal stem cells and found that the cells, under appropriate conditions, could convert into bone-forming osteocyte cells.
© 2021 Alshehri et al.
KAUST researchers, led by bioengineering professor Charlotte Hauser, had previously shown that their ultrashort peptides could be mixed with cells in the nozzle of a 3D printer to create a type of bioink that, once ejected, would instantly solidify into desired forms2. But it was unclear if the synthetic material could sustain the full complexity of bone development, which includes the adhesion, spread and differentiation of bone-specific stem cells, along with the incursion of blood vessels needed for nutrient transfer and metabolic-waste removal.
Alshehri put the platform to the test. She and Hauser, together with Ph.D. student Hepi Hari Susapto, fine-tuned the stiffness of their hydrogels by altering the concentration of peptides in their mix. Once they had the right mechanical properties for bone growth, the researchers seeded the scaffolds with bone marrow–derived mesenchymal stem cells. Inside these 3D constructs, the cells maintained their capacity for self-renewal and, under appropriate conditions, could convert into bone-forming osteocyte cells.
The KAUST team then went one step further. The researchers added cells taken from human umbilical veins to their mini-bone scaffolds and found that the material could support a dense network of blood vessel formation as well. “Our model successfully accommodated more than one cell type without any compromise on their viability,” says Alshehri, who now hopes to develop even more sophisticated bone tissue models for evaluation in animal models and, eventually, as a regenerative therapy for patients with bone disease.
Alshehri, Hauser and their colleagues are also looking to extend the platform into other medical applications, not just bone regeneration. “Since blood vessels are an integral part of native tissues,” Alshehri says, “the successful culture of endothelial cells in 3D within these hydrogels holds a great promise for tissue engineering applications in general.”
References
-
Alshehri, S., Susapto, H.H. & Hauser, C.A.E. Scaffolds from self-assembling tetrapeptides support 3D spreading, osteogenic differentiation, and angiogenesis of mesenchymal stem cells. Biomacromolecules 22, 2094–2106 (2021).| article
- Susapto, H.H., Alhattab, D., Abdelrahman, S., Khan, Z., Alshehri, S., Kahin, K., Ge, R., Moretti, M., Emwas, A. & Hauser, C.A.E. Ultrashort peptide bioinks support automated printing of large-scale constructs assuring long-term survival of printed tissue constructs. Nano Letters 21, 2719–2729 (2021).| article
You might also like
Bioengineering
Self-aware biosensors boost digital health monitoring

Bioengineering
Algae and the chocolate factory

Bioengineering
Smart patch detects allergies before symptoms strike

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities
Bioengineering
Promising patch for blood pressure monitoring

Bioengineering
Sensing stress to keep plants safe
Bioengineering
Building better biosensors from the molecule up
Bioengineering




