Bioengineering | Bioscience
Stray DNA fragments challenge CRISPR precision
Simple fixes of unintended large genetic insertions uncovered in genome editing offer improved safety and reliability.
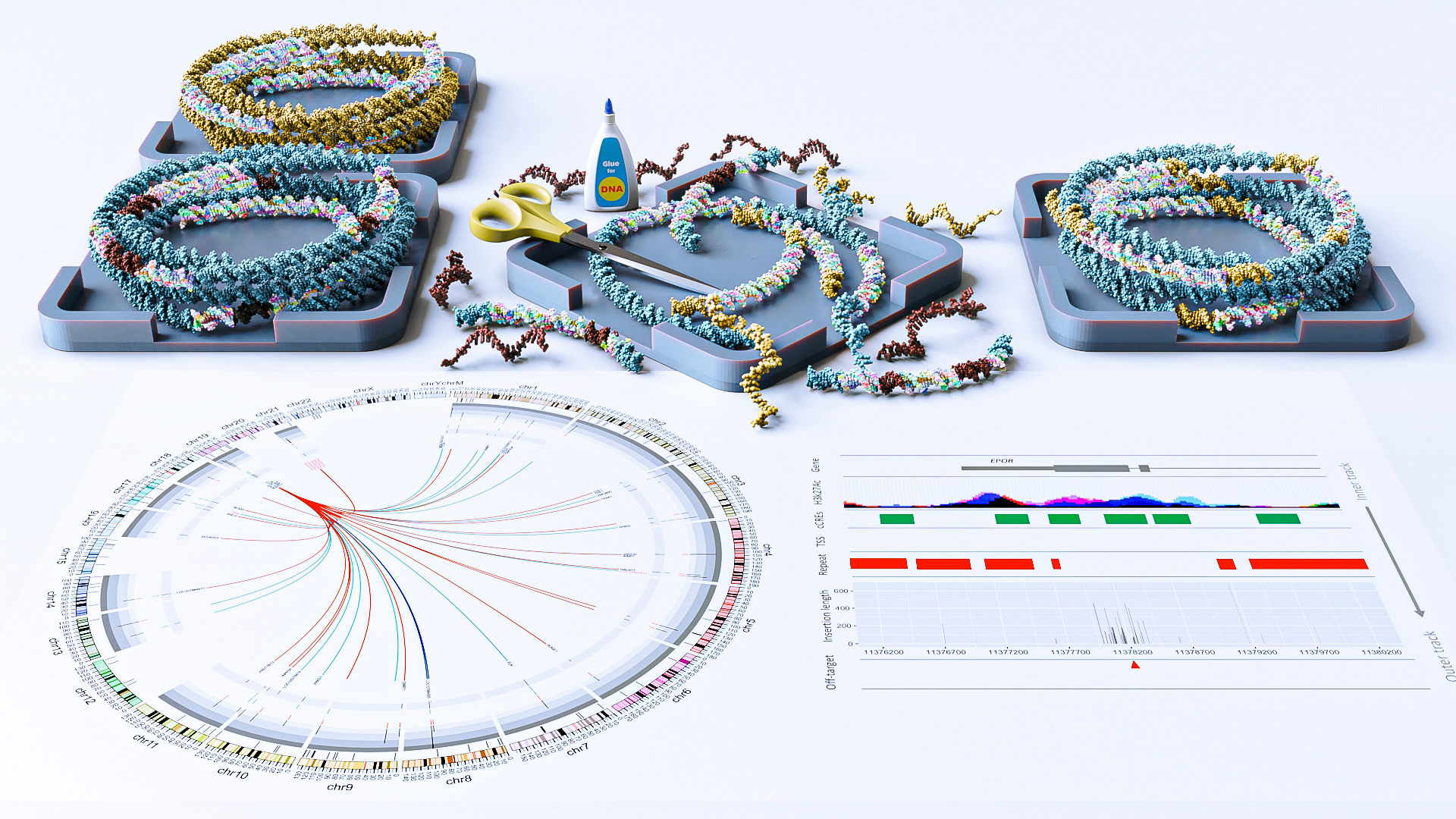
Imagine editing a manuscript, only to find random paragraphs from other books slipping into the text. That is the risk revealed in a new study of the celebrated genome-editing tool called CRISPR-Cas9: various unintended snippets of DNA sometimes get inserted in the edited region where they do not belong.
KAUST scientists uncovered this side effect in a detailed analysis of human embryonic stem cells edited with different types of donor DNA, whereby helper sequences provide a template for fixing the genome during the CRISPR process. They found that the genome editing platform, while efficient in cutting DNA, can sometimes leave behind large fragments of genetic material that were not part of the plan[1].
These stray pieces of DNA included repetitive sequences, regulatory elements and chunks from other parts of the genome. Though relatively rare — occurring in less than 1% of all edited cells — these large insertions could have significant consequences, especially in medical applications where accuracy is critical.
“This unexpected finding highlights the complexity of Cas9-editing outcomes,” says KAUST bioscientist Mo Li, who led the study.
Unlike large deletions, which typically result in the loss of gene functions, large insertions can lead to more complex and unpredictable genetic disruptions, including the activation of cellular pathways linked to cancer and other diseases. “While we cannot directly link these insertions to cancer risk yet, the presence of such sequences raises significant safety concerns for clinical applications,” Li says.
Li and his colleagues discovered that the type of donor DNA used plays a significant role in these outcomes. Linear donor DNA, for example, was more prone to causing unintended donor insertions compared to circular templates. The DNA repair process itself, which relies on the cell’s natural machinery, may also introduce these fragments when it mistakenly incorporates surrounding DNA into the repair site.
There is a simple fix. By chemically tweaking the donor DNA template with phosphorylation — a process that adds phosphate groups to the two ends of DNA — the researchers found that the likelihood of unintended large insertions dropped two-fold without compromising the efficiency of the intended edits.
“Phosphorylation of donor DNA is simple, does not alter experimental design or workflow, and reduces the risk of unintended structural variants without affecting gene-editing efficiency,” explains Chongwei Bi, a postdoc researcher in Li’s research group and the first author of the new study. “However, further validation is required in other settings to confirm the clinical impact and scalability of this approach.”
Without sounding alarms, the study authors emphasize the importance of refining CRISPR technology to make it even safer and more reliable — and of using ultra-sensitive DNA sequencing techniques like those developed in Li’s lab to systematically evaluate gene-editing products for rare unintended events.
“Additionally, designing editing strategies that minimize double-strand breaks or using alternative gene-editing tools without DNA breaks could further reduce these risks,” Li says. As gene editing moves into mainstream use, he points out, understanding and managing these subtleties will be crucial.
Reference
- Bi, C., Yuan, B., Zhang, Y., Wang, M., Tian, Y. & Li, M. Prevalent integration of genomic repetitive and regulatory elements and donor sequences at CRISPR-Cas9-induced breaks. Communications Biology 8, 94 (2025).| article
You might also like
Bioengineering
Self-aware biosensors boost digital health monitoring

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioengineering
Algae and the chocolate factory

Bioengineering
Smart patch detects allergies before symptoms strike

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience




