Material Science and Engineering
A cool approach to defect-free solar cells
Solar cells based on single-crystal perovskite films hit a new efficiency record of 21.9 percent.
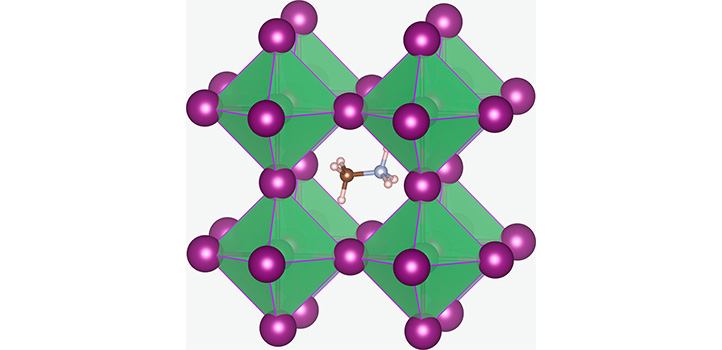
Chemists regularly use high temperatures to make things happen, but sometimes keeping cool can work better. Researchers at KAUST are discovering that a low-temperature approach can make better single crystals for use in solar cells.
Osman M. Bakr’s team in the KAUST Catalysis Center are finding many innovations in perovskites—materials that show great potential for building new generations of solar cells. Novel perovskites have positive and negative ions in the same arrangement as the natural perovskite calcium titanate (CaTiO3). Lead halide perovskites, containing lead ions and halide ions, such as chlorine and iodine in the perovskite mix, are attracting great interest for optoelectronic applications.
“Halide perovskite solar cells are considered the fastest growing photovoltaic technology,” says Abdullah Alsalloum, masters student and co-first author of the study.
The crystals have previously been generated at high temperatures, however these have produced troublesome defects. Now, the KAUST team has devised a solution, in both senses of the word, allowing better crystals to form.
Broadly, their “solvent engineering” approach modifies the liquid solvent in which the components of the perovskite are initially dissolved, and in which they combine to build the perovskite crystals.
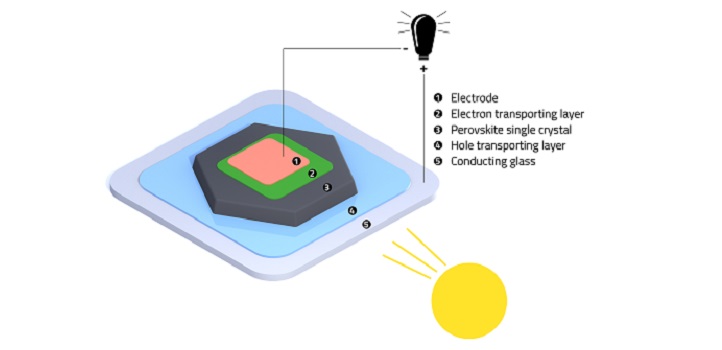
The structure of a perovskite solar cell that was created with a low temperature method.
© 2020 Abdullah Alsalloum
The specific perovskite they are developing is methylammonium lead iodide, containing large methylammonium ions (CH6N+), together with lead (Pb2+) and iodide (I–) ions. Previously, the solutions used to make the crystals needed to be maintained at 120 degrees Celsius, but such a high temperature interfered with the optimal incorporation of methylammonium and iodide ions into the structure.
“We investigated a variety of different solvents to carry the perovskite components and allow them to crystallize,” explains the other co-first author of the study Bekir Turedi. “Eventually, we found a solution that could work at less than 90 degrees Celsius and produce single crystals that were of significantly higher quality.”
The enhancement in crystal formation was matched by improved performance when the crystals were used as the light-absorbing layers in solar cells.
“We obtained markedly higher photovoltages, and power conversion efficiencies approaching 22 percent, among the highest ever reported for these perovskites,” says Bakr.
The researchers acknowledge that their single crystals are currently too small to be adopted for commercial use—a problem that currently hinders many other attempts to develop the full potential of perovskites in solar cells. Nevertheless, this work at KAUST takes an important step toward tackling this issue of size as well as on further improvements.
“The field is still in its infancy and the most exciting parts are yet to come,” Alsalloum concludes.
References
-
Alsalloum, A.Y., Turedi, B., Zheng, X., Mitra, S., Zhumekenov, A.A., Lee, K., Maity, P., Gereige, I., Alsaggaf, A., Roqan, I.S., Mohammed, O.F. & Bakr, O.M. Low temperature crystallization enables 21.9% efficient single-crystal MAPbI3 inverted perovskite solar cells. ACS Energy Letters 5, 657-662 (2020).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics




