Mechanical Engineering
Chemical enlightenment behind reflected shockwaves
Studying the wake of reflected shockwaves reveals the cascade of chemical reactions involved in combustion processes.
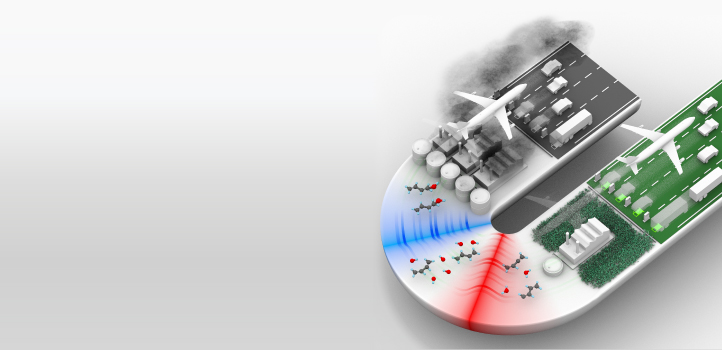
The hot, sometimes high-pressure, yet smooth conditions behind reflected shockwaves are the ideal environment for studying the chemical complexities of combustion.
Aamir Farooq, from KAUST’s Clean Combustion Research Center, and his team, have used a shock tube coupled with laser diagnostics to study the rapid cascade of chemical reactions set in motion when fuel combusts. This data can help in the design of cleaner, more efficient engines and fuels than are currently available. The insights gained could also be used to study reactions in the air that generate or remove pollutants, or even to study the atmospheric conditions of distant planets.
“Shock tubes are ideal chemical reactors, because of the nearly homogeneous zero-dimensional conditions behind reflected shockwaves,” Farooq says. The team shines lasers through the shock tube to monitor the chemistry taking place. “Laser diagnostics enable us to make in situ measurements of chemical species being consumed and formed during a chemical reaction,” Farooq adds. “We can track highly reactive free radicals, which play a key role in combustion kinetics.”
Two recent papers illustrate the breadth of information that can be acquired. Farooq and his team investigated the combustion chemistry of cyclic ketones, which can be made from plant waste and have excellent combustion behavior1. “Biomass-derived cyclic ketones attract interest due to their good antiknock property and their effectiveness in reducing harmful emissions,” says Dapeng Liu, a Ph.D. student in Farooq’s team. Reactions with hydroxyl radicals are one of the most important reactions initiating the combustion of cyclic ketones, but these had never been experimentally measured before at high temperatures.
“Compared with our measurements, researchers had overestimated the reactivity of cyclic ketone and hydroxyl reactions at high temperatures, but they had underestimated it for room temperature conditions,” says Liu. This new data will improve models used to design fuels incorporating cyclic ketones.
The team also studied hydroxyl reactions with diolefins, common molecules featuring two carbon-carbon double bonds2. This interaction is significant for fuel combustion, but also for atmospheric chemistry. “Isoprene, a well-known diolefin, is produced by animals and plants and can be found at high concentration in forest areas,” says Fethi Khaled, who recently completed his Ph.D. with Farooq.
“Our work showed the rich chemistry of hydroxyl radicals and diolefins,” Khaled says. The team revealed a clear shift from reaction pathways, where hydroxyl radicals add to diolefins at atmospheric conditions to pathways where hydroxyl radicals pluck hydrogen atoms from diolefins as temperatures increase up to combustion conditions.
The results add to the growing database of reaction rate coefficients the team has provided, which is used widely by chemical kinetic modelers and theoreticians to validate their calculations, says Farooq.
“In future, we are going to focus on radical plus radical reactions, which are much more challenging to study but play a critical role in many chemical environments,” he says. “We are designing new laser diagnostics to detect a wide array of molecules so that we can paint a complete picture of complex chemical reactions,” Farooq adds. “Finally, we are extending our methodologies to study reactions relevant to atmospheric chemistry and interstellar planets.”
References
-
Khaled, F., Giri, B. R., Liu, D., Assaf, E., Fittschen, C. & Farooq, A. Insights into the reactions of hydroxyl radical with diolefins from atmospheric to combustion environments. Journal of Physical Chemistry A 123, 2261 (2019).| article
- Liu, D., Giri, B. R. & Farooq, A. Cyclic ketones as future fuels: Reactivity with OH radicals. Journal of Physical Chemistry A 123, 4325 (2019).| article
You might also like

Mechanical Engineering
Cracking clean fuel combustion

Mechanical Engineering
Tiny sensor could transform head injury detection
Mechanical Engineering
Electrocatalytic CO2 upcycling excels under pressure
Chemical Engineering
Rethinking machine learning for frontier science

Mechanical Engineering
Falling water forms beautiful fluted films
Mechanical Engineering
Innovative strain sensor design enables extreme sensitivity
Mechanical Engineering
Turbulent flow shows surprise patterns that could help boost efficiency

Mechanical Engineering




