Material Science and Engineering
Single-electrode material streamlines functions into a tiny chip
Ruthenium oxide is used to integrate energy-storing microsupercapacitors and thin-film electronics at the transistor level.
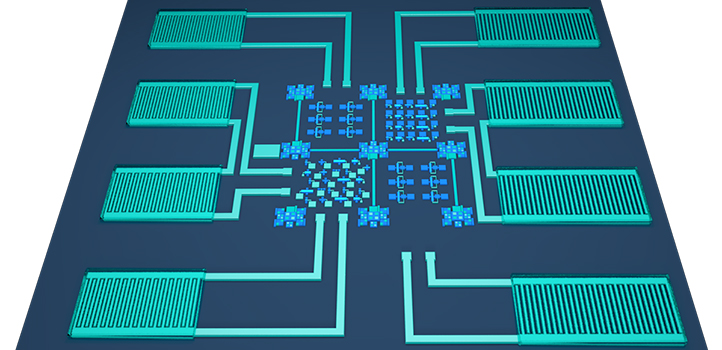
The ability to combine many functions into a single microchip is a significant advance in the quest to perfect the tiny, self-powered sensors that will expand the Internet of things. KAUST researchers have managed to combine sensing, energy-harvesting, current-rectifying and energy-storage functions into a single microchip.
“Previously, researchers had to use bulky rectifiers that converted intermittent harvested electrical energy into steady direct current for storage in electrochemical microsupercapacitors,” says Mrinal K. Hota, research scientist at KAUST and lead author of the study.
Hota explains that the key to integrating everything into a single chip was the development of ruthenium oxide (RuO2) as the common electrode material connecting all devices in the microcircuits. The team envisages a broad range of applications from monitoring personal health indications directly from the human body to environmental and industrial sensing.
“Our achievement simplifies device fabrication and realizes significant miniaturization of self-powered sensor devices,” says project leader Husam Alshareef.
A thin-film chip with the energy-storing microsupercapacitors arrayed along top and bottom of the chip. © 2019 KAUST
The ruthenium-oxide contacts are laid onto a glass or silicon substrate to connect sensing, energy-harvesting and current-rectifying electronics with one or more electrochemical microsupercapacitors that store the electrical energy. This creates a tiny system that can operate without any battery power. Instead, it uses available body movement or machinery vibrations as the reliable and continual source of energy.
“Unlike a battery, electrochemical microsupercapacitors can last for hundreds of thousands of cycles rather than just a few thousand,” Hota points out. They can also deliver a significantly higher power output from a given volume.
A key to creating electrode material suitable for connecting all devices was to make optimal ruthenium-dioxide surfaces with controlled roughness, defects and conductivity. These features enabled the team to use RuO2 for both electronics and electrochemical microsupercapacitors.

A schematic illustration of the integrated circuit fabricated on a one-inch glass substrate. The chip combines electronics and on-chip energy-storage units.
© Reproduced with permission from reference one © 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim
Another crucial innovation was to use a gel that, after application, solidifies into the supercapacitors’ electrolyte. This is a material that transports electric charge in the form of ions. The solidified gel was chosen to avoid any damage to rectifiers and thin-film transistors.
The researchers now plan to work to optimize the RuO2 electrodes further and explore linking many different types of sensors into their chips. They also want to investigate adding wireless communication into the device. This would allow biosensors and environmental sensors to send data remotely to any wireless receivers, including mobile phones and personal computers.
The team, led by Husam Alshareef, also includes Khaled Salama and Z.L. Wang from the Georgia Institute of Technology in Atlanta, USA.
References
- Hota, M.K., Jiang, Q., Wang, Z., Wang, Z.L., Salama, K.N. & Alshareef, H.N. Integration of electrochemical micro-supercapacitors with thin-film electronics for on-chip energy storage. Advanced Materials 31, 1807450 (2019).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics




