Bioscience
Visualization of brain structures helps to model function
State-of-the-art techniques and software help researchers recreate cellular brain structures and model biochemical processes that support the brain’s energy demands.
Computer simulations and virtual reality are used by KAUST researchers and collaborators in France to visualize the energetic coupling between neurons and astrocytes and to improve understanding of brain metabolism.
The human brain uses more energy than any other organ, accounting for around 20 percent of all glucose-derived energy. Neurons can’t meet their own energy requirements: they depend on supporting glial cells and the neurovascular system to supply various forms of sugar fuel. However, it is still unclear how this neuro-glio-vasculature network manages the brain’s energy demands.
Lactate is a fuel that neurons rely on for their energy needs. It is produced by astrocytes—the most abundant glial cell type in the central nervous system—and is shuttled to neurons. KAUST researchers, in collaboration with colleagues working on the Blue Brain Project at the École Polytechnique Fédérale de Lausanne, have now published the four-stage process they are following to develop computer simulations of the energetic coupling between neurons and astrocytes.
Realistic simulations require an understanding of the cellular space in which the biochemical reactions that manage energy supply take place. “To make biologically accurate models, we employ serial block-face electron microscopy to image thousands of serial sections from rodent brains and use them to make 3D models of astrocytes,” explains Corrado Calì, a researcher in Pierre Magistretti’s bioscience team at KAUST. These reconstructions reveal the strategic location of energy stores and power sources (mitochondria) in astrocytic compartments to improve the cells’ energetic efficiency.
Developing bespoke analytical tools to describe the astrocyte’s morphology mathematically enables the researchers to incorporate this information into computer simulations. “This represents a major step forward because, to date, 3D models have mostly been used for qualitative rather than quantitative analysis,” says Calì.
Another unique aspect of this study is the pioneering use of virtual reality (VR) in neuroscience. The resulting models are difficult to analyze on a 2D screen, so to make them more accessible, the researchers at KAUST’s Visual Computing Center embedded them in a VR analytical framework. “We have produced tools for the visual analysis of data extracted from electron microscopy images that help understand brain-energy metabolism in various parts of cells,” says Marco Agus, a computer scientist in Markus Hadwiger‘s group in the Center.
Although this study focused on brain-energy metabolism, the process developed by the authors can be applied to any cellular grouping to gain knowledge on other fundamental neural functions, such as learning and memory formation, at the molecular, cellular and network level.

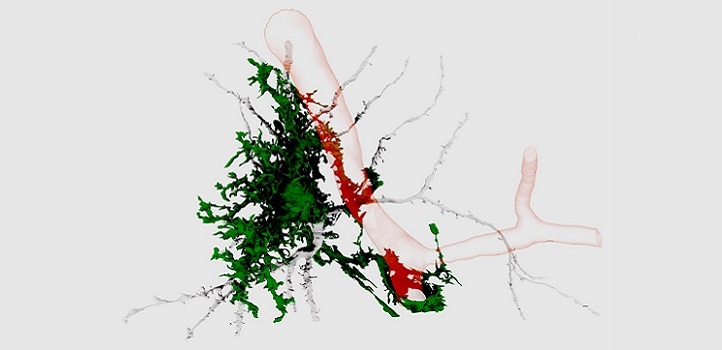
3D reconstructions from serial-section electron micrographs showing one astrocyte (green) interfacing with a blood vessel (red).
© 2018 Corrado Calì
References
-
Coggan, J.S., Calì, C., Keller, D., Agus, M., Boges, D., Abdellah, M., Kare, K., Lehväslaiho, H., Eilemann, S., Blaise Jolivent, R., Hadwiger, M., Markram, H., Schürmann, F. & Magistretti, P.J. A process for digitizing and simulating biologically realistic oligocellular networks demonstrated for the Neuro-Glio-Vascular ensemble. Frontiers in Neuroscience. 12, 664 (2018).| article
You might also like

Bioengineering
Bio-inspired network structures for next-generation AI

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities

Bioscience




