Bioscience
Spotting cancer’s greasy fingerprints
Laser-based microscopes can tune into multiple biomolecular signatures found in cancer cells.
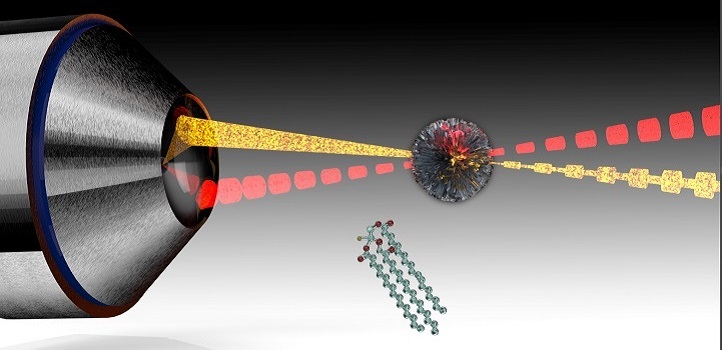
Laser-based microscopes can tune into multiple biomolecular signatures found in cancer cells.
Lipid droplets normally involved in storing fats have recently been revealed as key players in many cancer cell processes, including their ability to resist treatment. These lipids can now be quantitatively identified more easily than ever using a microscopy technique developed at KAUST.
The chemical bonds holding molecules together produce unique vibrations when excited by a light source. Researchers are now exploiting this effect to characterize living cells and tissue through stimulated Raman scattering microscopy. In this approach, ultrafast laser pulses are used to vibrate biomolecules throughout the volume of a sample. The resulting signals can then help visualize components, including lipids at speeds suitable for video imaging.

Siarhei Laptenok (left) and Carlo Liberale discuss the capabilities of their electronically tunable optical filter.
© 2020 KAUST
One challenge with stimulated Raman scattering involves collecting data from two significant parts of the molecules’ vibrational spectrum: a “C-H stretch” region, where hydrocarbon bonds elongate, and a “fingerprint” region, involving bond bending and twisting. Typically, researchers must take multiple measurements of each spectral zone with different optical components—a cumbersome process.
“The fingerprint region is the richest in detail that can reveal molecular structures, especially when it can be correlated with C-H stretch information,” explains bioscientist Siarhei Laptenok, a postdoc in Carlo Liberale’s group. “It’s critical to have the capability of collecting rapidly from both regions with a single measurement.”
To speed up the vibrational measurements, Liberale’s team incorporated an electronically tunable optical filter into their microscope. The filter selects the beam’s emissions on a very fast time scale that minimizes the need to perform time-consuming steps, such as changing or re-aligning optical components of the microscope. In this way, there is a dramatic fall in time for a typical high-resolution scan across the two main regions, from minutes to seconds, while retaining high spectral resolution.

Siarhei Laptenok (left) calibrates the laser setup with co-author Luca Genchi.
© 2020 KAUST
To demonstrate the biomedical potential of their microscope, the KAUST team collected vibrational data on human liver cancer cells. By selecting laser light that stimulates lipids, the researchers were able to produce images of the cancer cell that spotlight the tiny internal regions housing high concentrations of fat molecules through specific vibrational fingerprints. Other experiments also revealed the microscope could differentiate fats based on their saturated hydrocarbon content.
“Being able to see lipid droplets with great resolution and detail is really exciting, especially because it has only just come to light that cancer cells metabolize lipids in abnormal ways,” says Liberale. “We are interested in seeing how these droplets change when exposed to conditions, such as chemotherapeutic agents.”
References
- Laptenok, S.P., Rajamanickam, V.P., Genchi, L., Monfort, T., Lee, Y., Patel, I., Bertoncini, A. & Liberale, C. Fingerprint-to-CH stretch continuously tunable highspectral resolution stimulated Raman scattering microscope. Journal of Biophotonics e201900028 (2019).| article
You might also like

Bioscience
Robust workflow built for chemical genomic screening
Bioscience
Cell atlas offers clues to how childhood leukemia takes hold
Bioscience
Hidden flexibility in plant communication revealed
Bioscience
Harnessing the unintended epigenetic side effects of genome editing
Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities
Bioscience
AI speeds up human embryo model research
Bioscience




