Chemical Engineering
Crystal clear solvent filtration
Covalent organic crystal networks generate high-selectivity and high-flux membranes for organic solvent filtration.
Covalent organic materials with well-ordered porous microstructures could provide the membranes needed for technology to meet increasingly stringent environmental controls and be cost effective to produce.
Researchers from KAUST have generated crystalline membranes, consisting of organic building blocks held together by covalent bonds, that enable organic solvent purification and recovery with high selectivity and high flux. The membranes also have potential for innovative processes in the chemical industry.
Organic solvent nanofiltration typically involves polymer-based membranes that feature tiny pores but form dense and amorphous networks. Well-ordered microporous materials, such as zeolites and metal-organic frameworks, perform significantly better than these conventional membranes in various separation processes. However, they are not suitable for extensive use in liquid separation because of their poor structural and chemical stability in liquids.
Now, a team led by Zhiping Lai, has developed a synthetic approach that produces well-ordered microporous materials that are stabilized by covalent keto–enamine linkages. These linkages are produced from the reaction between amine and aldehyde functional groups of organic compounds.

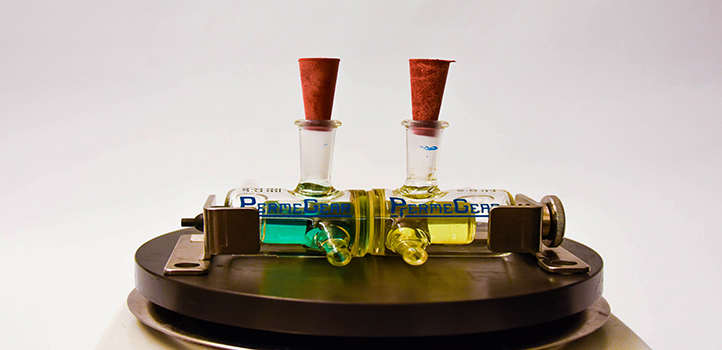
Zhiping Lai (left) holds up the team’s high-flux membrane for organic solvent filtration with first author of the paper, Digambar Shinde (right).
© 2019 KAUST
The researchers fabricated the membranes by the Langmuir–Blodgett method, which reliably produced large-area thin films of well-defined thickness using amphiphilic aldehyde and amine precursors. They deposited the precursor mixture solutions on a water surface to form weakly bound two-dimensional hexagonal structures. Once the solvent evaporated, they compressed the films laterally and added an organic acid to the mixture, transforming the reversible bonds into covalent keto-enamine linkages and sealing the hexagonal structures in place.
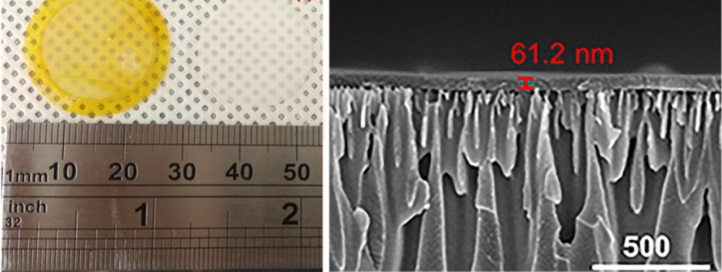
The researchers synthesized high-flux, high-selectivity solvent nanofiltration membranes (yellow, left) from a covalent, organic, porous material (right).
Reproduced with permission from reference 1 © 2018 The American Chemical Society
The new membranes outperformed amorphous analogs fabricated using the same method and the best polymer-based systems. “They share the same chemistry as polymer analogs, resulting in similar hydrothermal, chemical and mechanical stabilities, but their fluxes are higher,” says postdoc, Digambar Shinde, first author of the paper.
The organic solvent permeability of the new membranes is almost an order of magnitude higher than that of the best-reported polymer membranes, he adds. The membranes were more stable than metal-organic frameworks and more cost-effective than inorganic membranes. They could also separate mixtures of dye molecules differing in molecular weights and sizes.
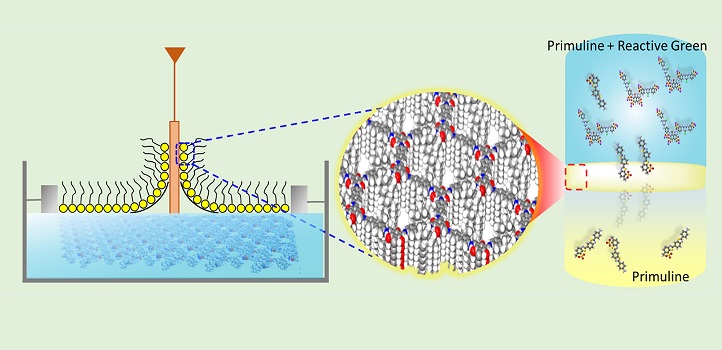
During the Langmuir–Blodgett process (left), the amine and aldehyde molecules gradually formed an extended two-dimensional network at the air–water interface (middle). The network consisted of amine and aldehyde molecules paired into hexagonal structures (right).
© 2018 Digambar Shinde
The team is currently working on extending the use of the membranes to a multitude of applications. “The pore sizes of these membranes are suitable for seawater desalination pretreatment, food processing, purification of pharmaceutics and medical processes, such as hemodialysis,” says Shinde. The membranes can also be useful for eliminating heavy metals, viruses and bacteria.
References
- Shinde, D.B., Sheng, G., Li, X., Ostwal, M., Emwas, A.-H., Huang. K.-W. & Lai, Z. Crystalline 2D covalent organic framework membranes for high-flux organic solvent nanofiltration. Journal of the American Chemical Society, 140, 14342−14349 (2018).| article
You might also like

Chemical Engineering
Urban air pollution goes up in smoke
Chemical Engineering
Rethinking machine learning for frontier science

Chemical Engineering
Magnetic nanoparticles capture microplastics from water

Chemical Engineering
Biogas upgrading goes with a swing

Chemical Engineering
Stronger, lighter, cheaper: a new route to carbon fiber production

Chemical Engineering
Unveiling the role of biomass-burning aerosols in atmospheric reactions

Chemical Engineering
Slashing industrial emissions using a hybrid model approach
Chemical Engineering




