Chemical Engineering | Environmental Science and Engineering
Precision separations with perfect pores
Nanofiltration membranes with bespoke porous architectures enable ultra-selective and energy-efficient separation of complex mixtures.
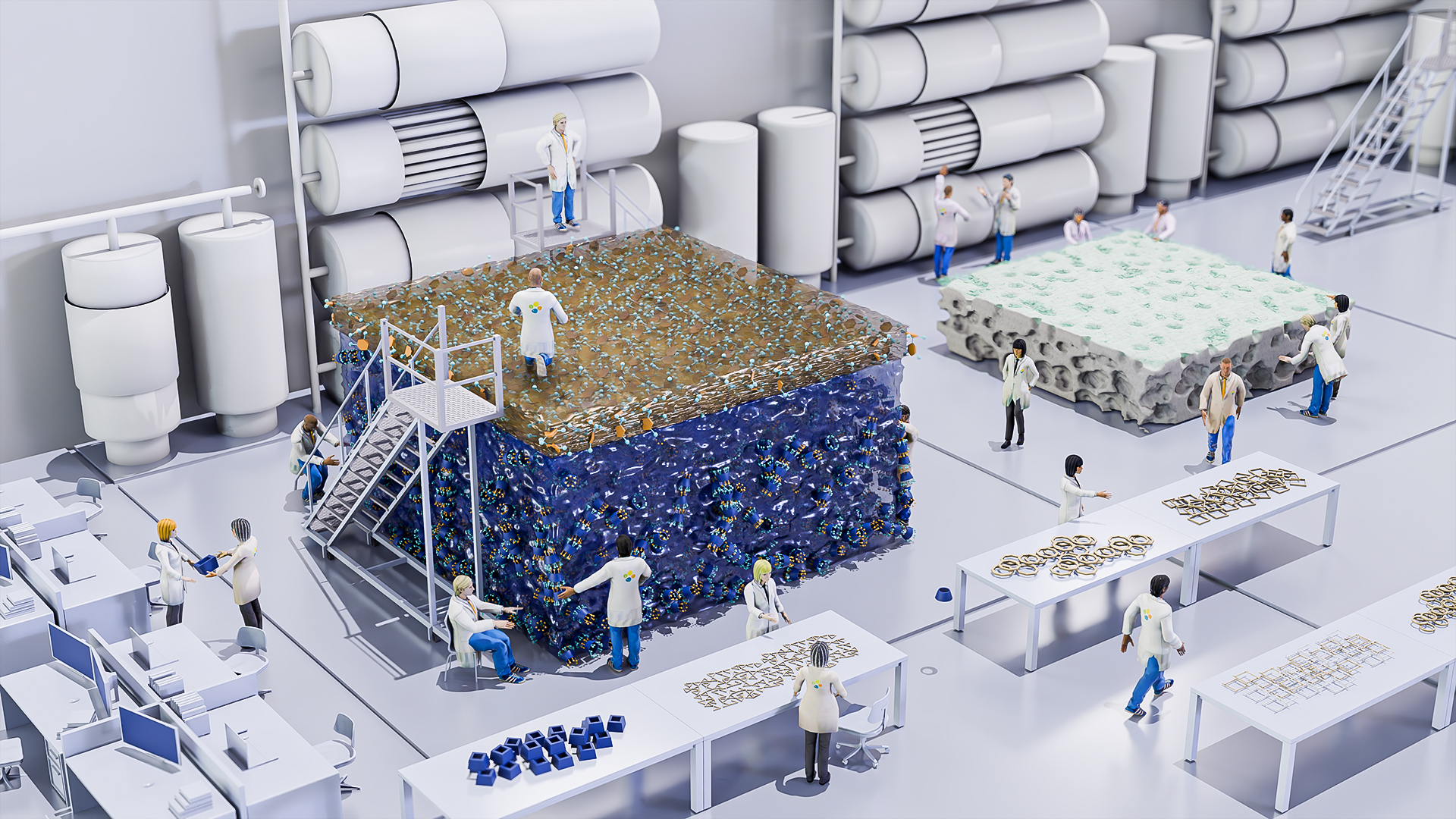
High value metals, such as lithium, could be extracted directly from seawater, lake brines, or could be recycled from electronic waste, a study of designer nanoporous membranes suggests. The membranes incorporate ring-shaped ‘macrocycle’ molecules, which form precisely defined pores that permit only the target metal to pass[1]. Macrocycle membranes can also efficiently purify challenging mixtures of high value chemicals, such as pharmaceutical ingredients, the KAUST research team has shown[2].
Separating multicomponent mixtures is a core part of industrial activity ranging from raw minerals processing to fine chemical and pharmaceutical production. These steps have a large environmental footprint, however. Most separations involve energy-intensive heat-driven processes such as distillation and evaporation. “More effective separation methods would lead to a much more sustainable and profitable chemical industry, reducing the need for carbon capture at the end of the process,” says Suzana Nunes, who led the research.
“It is crucial to develop new materials that enable more efficient separations,” says Gyorgy Szekely, who co-led part of the work. A few industries – notably, seawater desalination – have employed membranes as an energy-efficient alternative to heat-driven separations. The membranes they use consist of tightly woven thin polymer sheets, through which water molecules can squeeze but salt cannot.
“Commercial membranes mostly separate water from salt, or separate large solutes from very small ones,” Nunes says. But because these membranes lack precisely defined pores, they are ineffective for finer-grained separations, she adds.
To efficiently separate mixtures of similarly sized molecules, Nunes and her colleagues developed membranes that incorporate ring-shaped macrocycles into their structure. “The macrocycles can act as a pore, tuned specifically for the size of molecule or ion to be transported,” Nunes says.
The team’s first challenge was to develop a versatile and scalable method for making membranes featuring embedded macrocycle, Szekely notes. The researchers were able to adapt an existing method that industry already uses to manufacture membranes, called interfacial polymerization.
This highlights the team’s pragmatism to enable easy industrial uptake. “Our focus is to invest in methods and materials that could fabricated at the scales required by industry,” Nunes says.
The membrane was made by combining the macrocycle component with a molecular linker. The interfacial polymerization method brings these two components together under controlled conditions, by exploiting that water and organic solvents do not mix. When the team created a macrocycle component that dissolved in water, and a linker component dissolved in organic solvent, then combined the two immiscible liquids, the membrane self-assembled as a thin film at the interface between the two liquids.
Nunes and Szekely showed that when they made membranes from a macrocycle called 18-crown-6, they could separate mixtures of closely related pharmaceutical molecular ingredients. “The membranes showed excellent selectivity,” Szekely says. “We could separate solutes that have small difference in their molecular weight, which is highly sought-after by the pharmaceutical and fine chemical industries.”
Nunes also showed that, using a macrocycle called a cyclodextrin, the membrane could selectively concentrate valuable lithium or magnesium from salty mixtures mimicking seawater and industrial brines.
Macrocyclic molecules are readily available in a wide range of sizes, suggesting that ultra-selective macrocycle membranes tailor made for specific industrial separations could be created. Mixtures of metals generated from electronic waste recycling could also be separated by this method, Nunes says. “We are now investigating different macrocycles, different forms of self-assembly and different applications,” Nunes says. “In collaboration with industry, we are also working on scaling up the membranes,” she adds.
Reference
- Hong, S., Di Vincenzo, M., Tiraferri, A., Bertozzi, A., Górecki, R., Davaasuren, B., Li, X., Nunes, S. P. Precision ion separation via self-assembled channels. Nature Communications 15, 3160 (2024).| article
- Alhazmi, B., Ignacz, G., Di Vincenzo, M., Hedhili,M. N., Szekely, G., Nunes, S. P. Ultraselective Macrocycle Membranes for Pharmaceutical Ingredients Separation in Organic Solvents. Nature Communications 15, 7151 (2024).| article
You might also like

Chemical Engineering
Urban air pollution goes up in smoke
Chemical Engineering
Rethinking machine learning for frontier science
Environmental Science and Engineering
Bacteria reveal hidden powers of electricity transfer

Environmental Science and Engineering
Wastewater surveillance tracks spread of antibiotic resistance

Bioscience
Super fungi survive extreme Mars-like environments

Environmental Science and Engineering
Rethinking food systems to restore degraded lands

Environmental Science and Engineering
Combat climate change by eliminating easy targets

Chemical Engineering




