Bioscience
The dynamic molecular changes of homing
Molecules reorganize to slow stem cells down, giving them a chance to adhere to and eventually cross the inner lining of blood vessels into the bone marrow.
A new microscopic technique allows researchers to observe the initial nanoscale changes that are part of the complex process of transplanted stem cells homing in on, and specifically settling in, the bone marrow. Understanding the details of this process can improve the success of stem cell transplantations for treating leukemia.
KAUST microscopist Satoshi Habuchi and colleagues developed their microfluidics-based super-resolution microscopy platform to improve the ability of researchers to visualize the dynamic nanoscale changes that happen to stem cells as they move in blood. Current techniques only enable researchers to identify the molecules involved in stem cell homing—a phenomenon that allows cells to migrate to their organ of origin—but not what is happening at the nanoscale or over time.
For homing to succeed, stem cells moving along in a flowing circulation need to be able to slow down, attach to the inner lining of the blood vessel (called the endothelium), and then migrate across this lining into the bone marrow.
This process is important for treating leukemia patients: injected stem cells that migrate to the bone marrow can make new, healthy blood cells after the cancerous leukemic cells are destroyed by chemotherapy. However, stem cell homing in these transplantations is often inefficient and thus requires large numbers of stem cells to improve their chances of reaching the bone marrow.
“Understanding the molecular process will directly contribute to increasing homing efficiency and will eventually contribute to the success of leukemia treatment,” explains KAUST alumna Karmen AbuZineh, first author of the study.
Habuchi and his team’s approach involves fixing the stem cells as they roll and interact with endothelium molecules that are immobilized at the bottom of the team’s microfluidic chamber. The molecules are tagged with fluorescent antibodies so they can be visualized under a super-resolution fluorescent microscope.
The approach showed that, as stem cells move through blood vessels, CD44 molecules, located on protrusions extending from their membranes, reorganize themselves with the help of the cytoskeletal structural protein actin and fatty-acid chains of lipids. This reorganization helps the stem cells bind strongly to a molecule called E-selectin on the inner lining of blood vessels, slowing them down so they can roll over the vessel endothelium and eventually bind to it.
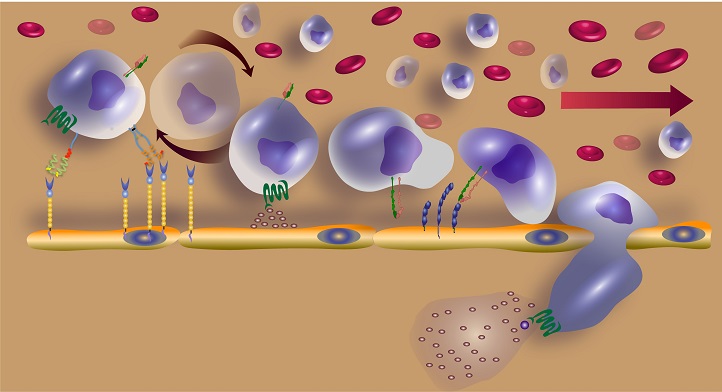
The initial tethering and rolling step of stem cell homing is mediated by molecules, including E-selectin, on the inner surface of blood vessels and CD44 on the surface of stem cells.
© 2018 Jasmeen Merzaban
“Our new method revealed that the molecular dynamics of hematopoietic stem-cell homing are far more complicated than previously thought,” explains AbuZineh. “Our study yielded more questions than answers,” she says, and reveals details about the initial step of homing. “This is just the beginning.”
The team will now apply their technique to investigate the subsequent steps involved in homing.
References
-
AbuZineh, K., Joudeh, L. I., Al Alwan, B., Hamdan, S. M., Merzaban, J. S. & Habuchi, S. Microfluidics-based super-resolution microscopy enables nanoscopic characterization of blood stem-cell rolling. Science Advances 4, eeat5304 (2018).| article
You might also like

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities

Bioscience
AI speeds up human embryo model research

Bioscience




