Material Science and Engineering
A metallic meeting in the middle
The interface between two tin-oxide semiconductors can exhibit unexpected metallic properties.
Metal oxidation is harnessed in many industrial applications. KAUST researchers have modeled the boundary between two metal oxides to reveal their metallic properties, which could lead to positive applications in electronics.
Our familiarity with rust, which occurs through the oxidation of iron to make it flaky and weak, means we usually consider oxidation of metals to be detrimental. But some metal oxides are useful. For example, they have great potential in electronics because they can be both transparent and flexible. They can exhibit magnetic properties, which opens the door to high-performance, ultrafast computer memories. They can be sensitive to their environment, making them useful for gas sensors.
Recently, the potential of semiconducting tin monoxide (SnO) for electronic applications was revealed when KAUST scientists determined a record high mobility, which refers to how easily a charge-carrying particle can travel through the material. In this case, the charge carriers were not electrons, but holes. Holes behave very similar to electrons, but they carry a positive rather than a negative electrical charge.
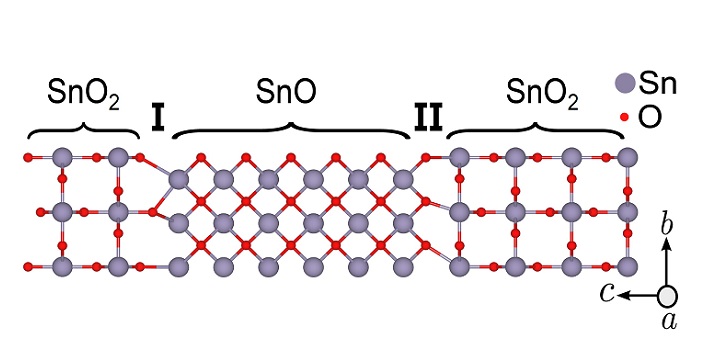
Obtaining pure tin monoxide is challenging because the fabrication process often also creates tin dioxide (SnO2). In general, the interface between two oxides can play host to a wide variety of exotic physics, from superconductivity to ferroelectricity, whereas the properties of the interface between tin monoxide and tin dioxide are largely unknown.
Arwa Albar, now an assistant professor at King Abdulaziz University, did this work as part of her Ph.D. studies at KAUST, together with Hassan Ali Tahini and her supervisor, Udo Schwingenschlögl. The scientists theoretically modeled the boundary between the two oxides using so-called density functional theory. With this technique they were able to determine the density of electrical charge at the interface for different atomic arrangements. They showed that the boundary can support freely moving holes in what is known as a quantum gas, which gives the interface a metallic character.
“The new model accurately predicts the amount of charge at the interface,” confirms Albar.
Quantum gases have already been identified at oxide interfaces in other material systems. They can arise due to a discontinuity in charge between two materials.
“The quantum gas formation is explained by a mechanism known as polar catastrophe in which the electrons arrange themselves to avoid a divergence in electrostatic potential,” says Schwingenschlögl. What is unusual about the tin monoxide–dioxide interface is that it lacks such a discontinuity in charge. “Instead, the amount of charge per interface area is different on the two sides of the interface,” explains Schwingenschlögl. “We call this ‘charge density discontinuity’ rather than the conventional ‘charge discontinuity’.”
The team predicts that this same phenomenon could also occur in other combinations of materials. “It will be necessary to investigate how the properties of the quantum gas can be controlled,” says Schwingenschlögl.
References
-
Albar, A., Tahini, H.A. & Schwingenschlögl, U. Metallicity at interphase boundaries due to polar catastrophe induced by charge density discontinuity. NPG Asia Materials 10, e469 (2018).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics




