Chemical Engineering
Setting regenerable polymer traps
Stable and recyclable materials synthesized using intrinsically porous polymers selectively retain CO2 from exhaust emissions and natural gas.
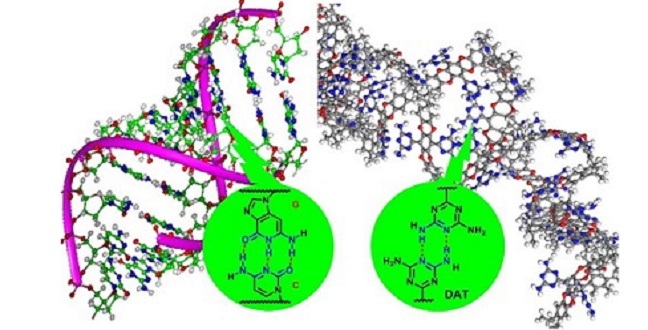
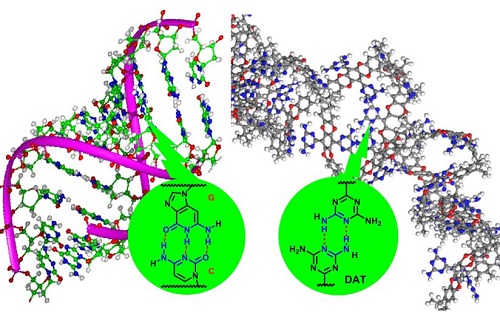
Hydrogen bonding in DNA (left) and between DNA-like functional groups in the adsorbent (right)
Reproduced with permission from ref 1© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Adsorbents that effectively isolate CO2 from other gases, such as nitrogen (N2) and methane (CH4), have been developed by KAUST using polymers with built-in porosity1. Such polymer-based materials would help to control CO2 levels released into the atmosphere and existing in natural gas.
Unlike their typical analogues, these polymers generate micropores because their rigid, ladder-type chain structure prevents dense packing.
Industrial facilities, such as oil refineries and natural gas processing plants, typically depend on amine scrubbing for CO2 removal. In this process, CO2-rich acid gas passes through an aqueous solution of highly reactive amines that transform and retain the pollutant in solution. However, this corrosive gas treatment requires a lot of energy and capital, which has restricted its large-scale use.
Adsorbents consisting of porous materials with regular structures, such as zeolites, metal-organic frameworks and mesoporous silicas, have emerged as attractive alternatives to amine scrubbing because of their large surface area and good selectivity. However, their pores are readily damaged and blocked from the build-up of heavy hydrocarbons in flue gas and natural gas, which depletes their adsorption capacity. When fouled or broken, these low-stability structures are difficult to restore.
KAUST Associate Professor Zhiping Lai and colleagues from the University’s Advanced Membranes and Porous Materials Center have created a recyclable polymer-based adsorbent that is stable under normal operational conditions. “If deteriorated or fouled, this structure can be easily disassembled to release the fouling agents and later regenerated through a simple solution-casting process,” said Lai.
The researchers produced their adsorbent by modifying an intrinsically porous polymer using a DNA-like functional group called DAT (see image). Showing stronger CO2 affinity, the adsorbent also displayed greater affinity with CH4 than N2 compared to its precursor.
According to Lai, the DAT group plays multiple roles here. It forms a hydrogen-bonded network of polymer chains that obstructs large pores and hinders the transport of N2-sized molecules. Its numerous Lewis base-like nitrogen atoms enhance the CO2 affinity while its aromatic components present attractive interactions with hydrogen atoms in CH4.
“Adsorbents have been a long-standing territory of inorganic materials,” said Lai. His team’s findings demonstrate that, besides their greater stability and ability to overcome fouling, organic polymers outperform conventional inorganic adsorbents. “Therefore, it is time to consider organic and hybrid materials for next-generation adsorbents,” he added
“Our adsorbent can be directly used in a well-established gas separation technology called pressure swing adsorption,” said Lai.
The researchers are now investigating ways to increase the adsorption capacity of their material.
References
- Wang, X., Liu, Y., Ma, X., Das, S. K., Ostwal, M…& Lai, Z. Soluble polymers with intrinsic porosity for flue gas purification and natural gas upgrading. Advanced Materials 29, 1605826 (2017).| article
You might also like

Chemical Engineering
Urban air pollution goes up in smoke
Chemical Engineering
Rethinking machine learning for frontier science

Chemical Engineering
Magnetic nanoparticles capture microplastics from water

Chemical Engineering
Biogas upgrading goes with a swing

Chemical Engineering
Stronger, lighter, cheaper: a new route to carbon fiber production

Chemical Engineering
Unveiling the role of biomass-burning aerosols in atmospheric reactions

Chemical Engineering
Slashing industrial emissions using a hybrid model approach
Chemical Engineering



