Environmental Science and Engineering
Extracting more from wastewater
A material made by combining a metal and a polymer can help generate hydrogen and fresh water from wastewater.
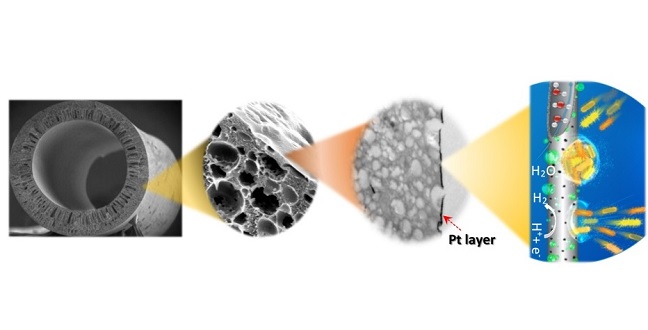
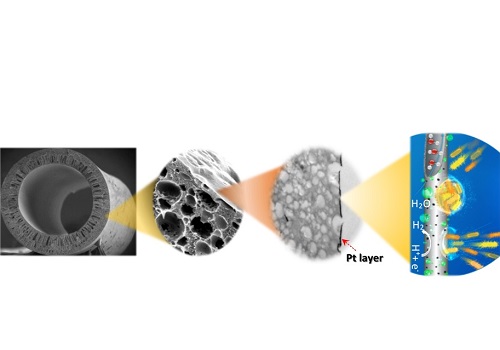
Coating a tube made of non-conductive polymer with a very thin layer of platinum is a simple option for a filtration membrane.
© 2016 KAUST
Fresh water scarcity and energy security are two critical global challenges facing us today. Researchers at KAUST have now created an advanced material that can address both problems simultaneously by producing clean water and hydrogen from wastewater1.
Electrochemical membrane bioreactors recover clean water for reuse and energy from wastewater by integrating micro- or ultrafiltration membrane cathodes with a microbial electrochemical system. This works by using a material full of pores small enough to block the passage of bacteria while allowing treated, clean water to pass through. The same material also acts as an electrode in an electrochemical circuit that recovers energy through the hydrogen-evolution reaction.
Previously, porous, flat electrodes have been used as both the cathode for the oxygen-reduction reaction and as the membrane to filter treated water; however, hollow fibers offer a greater surface-area-to-volume ratio, which improves the system’s performance. The drawback of this geometry is that these more complicated structures can be difficult to create and to optimize. For example, materials made from polymers are cheap to produce and flexible, but in general, because they act as electric insulators, they are not used as electrodes.
Suzana Nunes and Pascal Elias Saikaly from KAUST’s Biological and Environmental Sciences and Engineering Division and their colleagues from their Division and from the Nanofabrication Core Lab addressed this problem by coating a non-conductive polymer with a very thin layer of platinum, allowing the coated fiber to act as a catalyst for the hydrogen-evolution reaction.
The application of a uniform layer of metal catalyst on three-dimensional, thin, porous, polymeric, hollow fibers is difficult using traditional deposition techniques. To address this challenge, the team used atomic-layer deposition to exposehollow fluorinated polyoxadiazole polymer fibers to a 180 degrees Celsius gas containing platinum nanoparticles. “We produced the polymer fibers by simply adapting the phase-inversion method, which is already used in the industry,” explained Nunes.
Despite significantly lower platinum loading, the researchers confirmed that the hydrogen-evolution reaction of their material was similar to that of a platinum-carbon cloth, a material combination typically used for this application. Using atomic-layer deposition, the team could fine-tune the pore size of the hollow fibers, demonstrating effective reclamation of the treated effluent to yield high-quality water.
The team believes that the architecture of the three-dimensional porous, hollow fibers has broad applications. “These fibers can potentially be applied to directly convert carbon-dioxide waste from industrial sources to value-added products, such as methane and acetate, through microbial electrosynthesis,” explained Krishna Katuri, the study’s lead author.
References
-
Katuri, K.P., Bettahalli, N.M.S., Wang, X., Matar, G., Chisca, S., Nunes, S. & Saikaly, P.E. A microfiltration polymer-based hollow-fiber cathode as a promising advanced material for simultaneous recovery of energy and water. Advanced Materials 28, 9504-9511 (2016).| article
You might also like
Environmental Science and Engineering
Bacteria reveal hidden powers of electricity transfer

Environmental Science and Engineering
Wastewater surveillance tracks spread of antibiotic resistance

Bioscience
Super fungi survive extreme Mars-like environments

Environmental Science and Engineering
Rethinking food systems to restore degraded lands

Environmental Science and Engineering
Combat climate change by eliminating easy targets

Environmental Science and Engineering
Wastewater treatment to fight the spread of antibiotic resistance
Bioscience
Digging into the world of plant-growth-promoting microbes

Bioscience



