Material Science and Engineering
Here’s a tip for better stability
A new understanding of why tungsten-doped thin films degrade so rapidly in air may lead to better designs for semiconductor technologies.
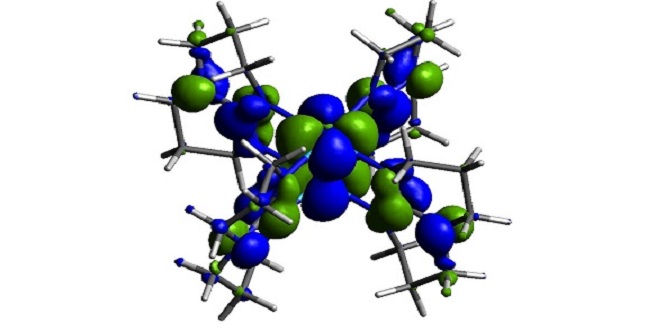
A W2(hpp)4 molecule—molecular doping is a key technique for flexible and low-cost organic semiconductor technologies.
Reproduced with permission from ref 1.© John Wiley & Sons.
A combination of experiments and theory have helped researchers at KAUST to improve the understanding of fundamental physics questions about molecular doping1—these will underpin the development of organic solar cells and field effect transistors.
Molecular doping is an essential method of creating flexible and low-cost optoelectronic devices such as organic solar cells and field effect transistors. The technique involves the controlled addition of impurities known as dopants into conjugated polymer thin films; this enables an otherwise electrically inert material to act like a semiconductor.
However, dopants that are electron-donating (n-type) can readily become oxidized if not housed under vacuum or inert atmosphere. This reduces device performance, which is a problem because many key processing steps are currently carried out under ambient conditions.
Max L. Tietze from the University’s Solar & Photovoltaics Engineering Research Center (SPERC) and colleagues have now examined this instability problem in more detail.
In 2013, the researchers demonstrated that by using organic semiconductors with sufficiently low energy levels, they produced highly stable n-type conductive films. Getting this result by using a di-tungsten complex as their n-dopant was a surprise, because in pure films a di-tungsten complex immediately degrades in air. At the time, the researchers offered two possible explanations for this phenomenon: one is single electron transfer from the dopant to the host material and the other is the formation of hybridized electronic states.
To gain deeper insight into the underlying mechanism, the researchers exposed four types of n-doped organic thin films to air. However, they observed the passivation effect only in films in which the host material’s electron affinity exceeds a critical value. Another strand of inquiry for the researchers was to use both conductivity measurements and photoemission spectroscopy to confirm the findings.
The researchers have now teamed up with the theory group led by Jean-Luc Bredas at KAUST. They showed that the formation of hybridized electronic host–dopant states can be excluded by numerical calculations, an effect that is owed to the three-dimensional (paddle wheel) structure of the di-tungsten complex.
Instead, it is conclusively shown that single electron transfer to oxygen–water complexes, which were supposed to constitute electron traps of an universal depth of 3.6 eV in conjugated polymers, actually governs the degradation of the n-doped films, and thus their passivation. Hence, Tietze’s team could identify a general limit for achieving air stable molecular n-type doping on a quantitative basis.
“This combination of experimental and high level theoretical work clearly addresses the most recent questions regarding the fundamental physics of molecular doping,” said Tietze.
References
- Tietze, M. L., Rose, B. D., Schwarze, M., Fischer, A., Runge, S. et al. Passivation of molecular n-doping: Exploring the limits of air stability. Advanced Functional Materials advance online publication, 3 March 2016. | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics



