Chemical Engineering
An easier path to treating malaria
Bringing three powerful chemical groups together offers new possibilities in drug development.
Drug developments need to keep up with the constantly evolving parasites that cause malaria.
© Stocktrek Images, Inc. / Alamy Stock Photo
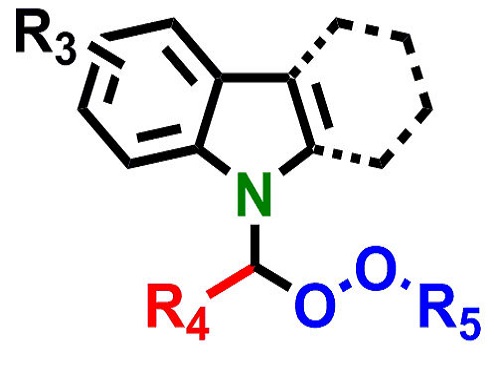
The crucial chemical structure at the heart of the potential new drugs.
© 2015 KAUST
A new chemical route for potential antimalarial compounds has been pioneered by chemists at KAUST. It offers hope for improved treatment of malaria, a disease that kills more than a million people each year.
“The parasites that cause the disease continuously evolve and become resistant to existing drugs, so we need to constantly design and synthesize new drugs to treat malaria,” noted Xinbo Wang, a chemist in Associate Professor Zhiping Lai’s group in the KAUST Physical Science and Engineering Division. The work could also lead to the development of new drugs against bacterial infections, cancer and other diseases.
A chemical structure called the alpha-amino peroxide group, which is found in existing anti-malarial compounds, is a good basis for building potential new drugs. However, there are significant problems with existing methods for making such compounds because they require difficult chemical conditions.
Lai and his colleagues devised a simple and efficient procedure1 and showed that the alpha-amino peroxide group can be combined with an “indole” chemical grouping to make the “N-(alpha-peroxy)-indole” structure.
“There are lots of reports on the synthesis of indole or peroxide-containing compounds, but there are no general methodologies for combining the two functional groups in one molecule that had been previously reported,” stated Wang.
The KAUST team took things a step further by adding a carbazole group. The indole, carbazole and peroxide groups occur separately in many antimalarial, antibacterial and anti-tumor compounds.
“Merging these functional groups in one molecular structure may offer great potential for the discovery of new types of drugs,” Wang said.
The reactions proceed in “one-pot, open atmosphere” conditions, meaning everything occurs in one vessel without complicated intermediate steps. The vessel is also open to the air rather than protected by unreactive gases, as is required for many similar chemical processes.
“Our procedure could be easily handled and scaled-up to allow the construction of a new library of molecules for drug screening,” he noted.
Upscaling is the challenge that the KAUST researchers plan to turn to next to make a wide variety of compounds that can be tested for useful biological activity against a variety of diseases, and especially against malaria.
In developing their new chemical procedures, the researchers drew inspiration from a chemical reaction used by another research project at KAUST.
“Our story proves the importance of a multidisciplinary research environment, which we consider is the treasure of KAUST,” Lai said. “Here, experts from different fields work closely together.”
References
- Wang, X., Pan, Y., Huang, K.-W. & Lai, Z. One-pot synthesis of N‑(α-peroxy)indole/carbazole via chemoselective three-component condensation reaction in open atmosphere. Organic Letters 17, 5630 – 5633 (2015). | article
You might also like

Chemical Engineering
Urban air pollution goes up in smoke
Chemical Engineering
Rethinking machine learning for frontier science

Chemical Engineering
Magnetic nanoparticles capture microplastics from water

Chemical Engineering
Biogas upgrading goes with a swing

Chemical Engineering
Stronger, lighter, cheaper: a new route to carbon fiber production

Chemical Engineering
Unveiling the role of biomass-burning aerosols in atmospheric reactions

Chemical Engineering
Slashing industrial emissions using a hybrid model approach
Chemical Engineering



