Computer Science
Noisy cells produce bursts of protein
A new mathematical model explains how random factors affect the production of proteins within the cells.

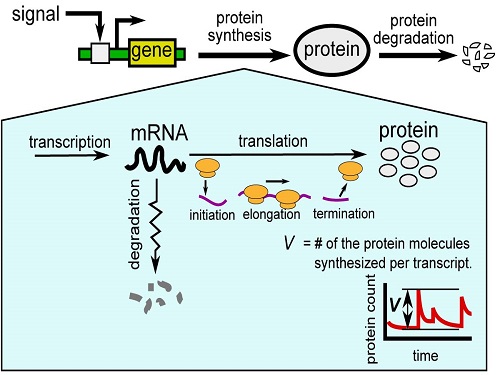
KAUST researchers model how gene expression “noise” affects protein production in bacterial cells.
Reproduced with permission from Ref 1© 2015 The Royal Society of Chemistry
KAUST researchers have developed a new mathematical model that makes the complex process of synthesizing proteins from the genes that encode them more predictable.
The multi-step synthesis pathway, which is mediated by RNA molecules, can be affected by random events, creating bursts of protein production. The KAUST model captures the factors that make this “noise” in protein production and that may provide insights into how genes are controlled and how they evolved1.
Protein synthesis consists of two main stages: “transcription” of the genetic code into messenger RNA (mRNA) molecules and “translation” of the mRNA into proteins.
Study lead author Xin Gao from the University’s Computational Bioscience Research Center (CBRC) explained that this is a stochastic process that “leads to variability in the abundance of gene products (RNAs or proteins) in a single cell through time or among genetically identical cells,” he said.
This variability can have far-reaching effects on the evolution and function of cells such as cancer cells and microbial pathogens—including their capacity for drug resistance.
“Bursts” of translation are thought to be the main cause of variability, especially in simple organisms like bacteria, where there are often only small amounts of mRNA. However, translation itself comprises multiple steps, including initiation, elongation and termination.
Previous models treated initiation as the only rate-limiting step in protein production, but this may not always be realistic.
“Our study was inspired by recent research showing that translational efficiency can be strongly affected by the elongation steps under stress conditions, such as when drugs are applied to pathogens,” noted Gao.
The KAUST team responded by developing the more sophisticated model that has the capacity to handle more general situations, including internal and external influences on translation. The model allows them to explore the different mechanisms regulating protein production and showed that the distribution of the translational burst can have a strong impact on the features and fitness of a cell.
The team anticipates that their model might provide insights into cells’ responses to stress, which rely on changes in protein abundance.
“We had planned to analyze the association between stress and gene expression noise using experimental data, but we found we needed the theoretical model that is more general than previous models,” Gao said.
The research might also be applicable to more complex organisms. “Recent studies have shown that stress conditions can have strong effects on translation in yeast and plants, and so protein burst size distributions could have effects on their protein abundance levels,” stated Gao.
References
- Kuwahara, H., Arold, S.T. & Gao, X. Beyond initiation-limited translational bursting: the effects of burst size distributions on the stability of gene expression. Integrative Biology 7, 1622-1632 (2015). | article
You might also like

Computer Science
Green quantum computing takes to the skies

Computer Science
Probing the internet’s hidden middleboxes

Bioscience
AI speeds up human embryo model research
Computer Science
Improving chip design on every level
Computer Science
Sweat-sniffing sensor could make workouts smarter

Computer Science
A blindfold approach improves machine learning privacy
Computer Science
AI tool maps hidden links between diseases
Bioscience



