Material Science and Engineering
Catching a glimpse of the double helix
Direct imaging of a single DNA molecule provides insights into fundamental biological processes.

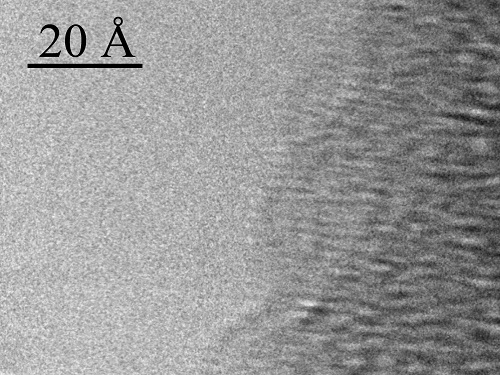
A HRTEM phase-contrast image of a single A-DNA helix bound to a 100 Å DNA bundle.
Reproduced with permission from Ref 1© 2015 This work is licensed under CC BY-NC .
Although the structure of DNA was first unraveled more than 60 years ago, a direct image of DNA’s detail has remained elusive. Researchers at KAUST have now developed a technique that allows them to visualize individual DNA molecules in real time1.
DNA has always been a slippery customer because of the low-contrast elements of which it is made and the fragile structure of the helical molecule. Now, an international team has perfected a preparation technique that fixes the unstable structure and an imaging process that minimizes its degradation.
According to senior author Enzo Di Fabrizio from the Physical Science and Engineering Division at KAUST, this opens potential to investigate molecular phenomena from DNA modification by mutation to DNA-protein interactions.
“Single molecule interactions are crucial for the understanding of many fundamental biological mechanisms,” he said.
The new process involves suspending single DNA molecules within a framework of minute silicon pillars. The DNA is then viewed with a high-resolution transmission electron microscope (HRTEM) that fires beams of electrons through it to produce a perfect image.
This method enabled the researchers to take key measurements of the DNA helix, including its diameter, the distance between the building blocks of the molecule (called “bases”) and the depth of the so-called “grooves” of the helix that provide a measure of its shape. In other words, said Di Fabrizio, “how the molecule is coiled.”
They could also determine the length of bonds between the two helices of DNA, which may indicate its level of methylation (a chemical effect that can alter gene activity) and pinpoint errors in base-pairing that may cause mutations and disease.
Di Fabrizio suggests that HRTEM images will be invaluable in figuring out exactly how DNA interacts with proteins in the cellular environment. He is focusing in particular on how repair proteins “dock” on to DNA to fix errors in the genetic code.
“Until now, this has not been seen at such a high resolution,” he said. “In the future, this will help scientists understand in detail how DNA repair occurs under both normal and pathological conditions.”
This is a rapidly-developing field—already there are cameras that are 100 times more sensitive than the one used in this work.
The team intends to combine their approach with a freezing technique known as “cryoEM” to achieve even greater detail and to extend it to imaging the structure of cell membranes.
References
- Marini, M., Falqui, A., Moretti, M., Limongi, T., Allione, M. et al. The structure of DNA by direct imaging. Science Advances 1, e1500734 (2015). | article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics



