Environmental Science and Engineering
The universal truth about sticky surfaces
Trapping molecules on custom-designed porous surfaces becomes easier with a new model that unifies previous theories of adsorption.

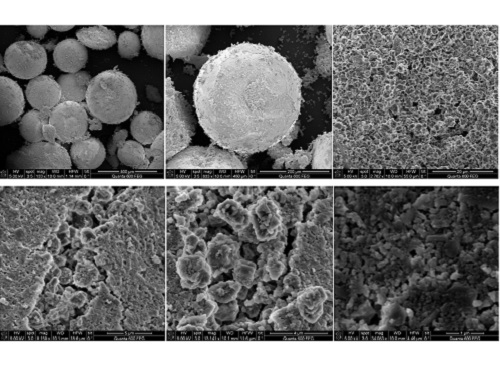
SEM images of the porous surface at increasing microscopy strength (from top left).
© 2017 KAUST
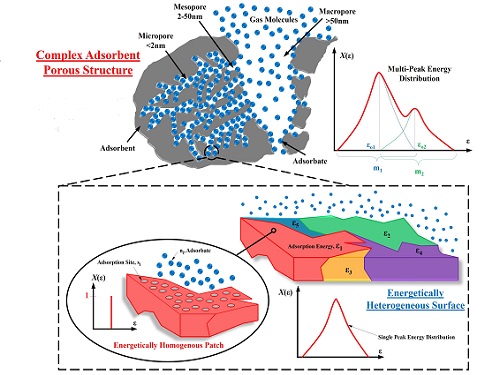
Differences in pore sizes on absorbent surfaces can be better understood with a model that spots tiny regions where gases attach at similar energies.
Reproduced with permission under creative commons licence from reference 1© 2017 KAUST
Many purification tools, from simple charcoal filters to complex desalination plants, rely on solids with millions of tiny pores to capture and remove contaminants without chemically binding to them. Now, a KAUST team has identified the key factors that connect adsorption on different kinds of porous surfaces, solving century-old problems of predicting uptake on unknown substances.
In the early 1900s, the concept of adsorption isotherms emerged to describe how adsorbents behave in the presence of steadily increasing amounts of molecules. These graphs have distinct shapes that depend on atom-scale surface properties—for example, whether particles stick in single layers or multilayers—and quickly became essential for designing and understanding purification setups. The majority of absorbents, chemists found, could be sorted into one of six isotherms after a few experimental measurements.
However, modern adsorbents with heterogeneous pore structures, such as metal-organic frameworks (MOFs), are proving more difficult to model. While these materials benefit from high-throughput testing of numerous samples, the need for individual isotherm measurements slows discovery considerably—a situation experienced by Professor Kim Choon Ng at KAUST’s Water Desalination and Reuse Center.
“We were working to improve treatment of seawater, and using isotherms was very tedious,” says Ng. “Everyone had to do their own trial and error work for particular applications, and there was no real theory to help people design absorbents.”
With researchers Muhammad Burhan and Muhammad Shahzad, Ng aimed to find out how the different isotherms could be combined into a single universal model. They proposed subdividing surfaces with nanometer-scale pore variations into tiny patches that adsorb guest molecules under similar thermodynamic and kinetic conditions. By introducing a probability factor to define the energy distribution of each patch, the team created a mathematical function capable of spotting significant characteristics of adsorbent surfaces.
Comparisons between predictions generated by the universal model and literature isotherms revealed the power of the new approach. Not only did the theoretical data match the measured experiments for all six isotherm categories, but multiple peaks appeared in the energy distribution plots when heterogeneous conditions are detected—parameters that may prove critical for development of innovative materials with fine-tuned sorption capabilities.
“Each adsorbent-adsorbate pair has its own distinct energy distribution function, which allows us to capture all the information in the isotherms,” explains Ng. “Materials scientists should be able to use techniques, such as acidification, to expand pore sizes in metal-organic frameworks and shift their energy distribution to increase uptake.”
References
- Ng, K.C., Burhan, M., Shahzad, M.W. & Ismail, A.B. A universal isotherm model to capture adsorption uptake and energy distribution of porous heterogeneous surface. Scientific Reports 7, 10634 (2017).| article
You might also like

Environmental Science and Engineering
Ultrathin water repellent membrane advances desalination
Environmental Science and Engineering
Bacteria reveal hidden powers of electricity transfer

Environmental Science and Engineering
Wastewater surveillance tracks spread of antibiotic resistance

Bioscience
Super fungi survive extreme Mars-like environments

Environmental Science and Engineering
Rethinking food systems to restore degraded lands

Environmental Science and Engineering
Combat climate change by eliminating easy targets

Environmental Science and Engineering
Wastewater treatment to fight the spread of antibiotic resistance
Bioscience




