Mechanical Engineering
The race for more efficient engines
New technique lays the foundation for greener transport fuels and next-generation engines.
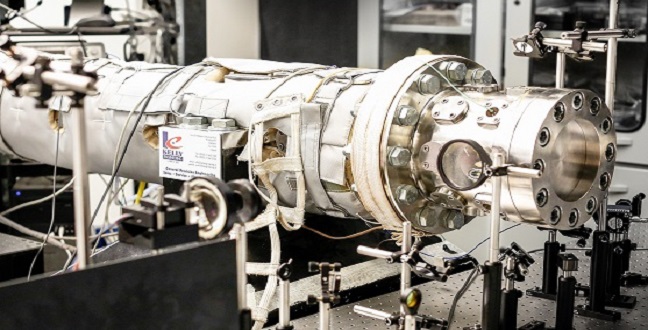
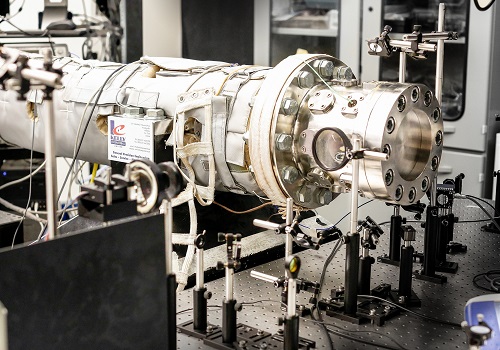
A shock tube used to model the auto-ignition properties of light naphtha.
© 2016 KAUST
The search for better engine performance powered by less polluting fuels will benefit from a new modeling technique developed at KAUST1.
Reducing the greenhouse gases released from the production and burning of fuels like diesel and gasoline —significant contributors to climate change—is a huge challenge facing the transportation industry.
Aamir Farooq, KAUST Associate Professor of Mechanical Engineering, and colleagues from the KAUST Clean Combustion Research Center worked with the Fuel Technology Team from Saudi Aramco and used an innovative technique for testing the properties of light naphtha, a fully blended low-octane highly paraffinic fuel. Farooq explained that this technique could open the potential for more advanced engines to run on fuels that release fewer greenhouse gas emissions.
“Optimizing fuel performance and engine designs must go hand-in-hand if we want to reduce emissions and improve engine efficiencies,” Farooq said. “Although gasoline and diesel engines have their advantages, we need a new type of engine that utilizes the strengths—while avoiding the drawbacks—of current engine technologies.”
Compression-ignition and spark-ignition engines, commonly referred to as diesel and gasoline engines, have fuel efficiencies of only 35 to 40 percent. A new engine design called a gasoline compression-ignition (GCI) engine, however, promises efficiencies of up to 55 percent as well as releasing fewer harmful soot and nitrogen oxides compared to current engines.
However, realizing these benefits requires a fuel with auto-ignition properties somewhere between gasoline and diesel.
“The auto-ignition properties determine the performance of the engine and so have a significant influence on the engine’s design,” noted Farooq. “Like all fuels, light naphtha contains hundreds of compounds, so to perform detailed engine optimization, we need a simpler model of the fuel that is called a surrogate fuel.”
The researchers compared auto-ignition properties of light naphtha with two surrogates—a simple primary reference fuel surrogate and a more complex surrogate—over a range of temperatures, pressures and fuel/air ratios. Operating at high temperatures, they first used a shock tube to study the auto-ignition behavior of the surrogates over different pressures. However, at lower temperatures reactions take longer, so a rapid compression machine was used to extend the observation time for the reactions.
They found the complex surrogate almost perfectly matched the light naphtha, and in particular at lower temperatures, providing a full picture of its auto-ignition characteristics.
“Our work has produced the first auto-ignition model for light naphtha, and it is being used by Saudi Aramco’s Research Center in Detroit to optimize GCI engine designs,” Farooq said.
References
- Javed, T., Nasir, E.F., Ahmed, A., Badra, J., Djebbie, K., Beshir, M., Ji., W. Sarathy, S. M. & Farooq, A. Ignition delay measurements of light naphtha: A fully blended low octane fuel. Proceedings of the Combustion Institute 36, 315-322 (2016).| article
You might also like

Mechanical Engineering
Cracking clean fuel combustion

Mechanical Engineering
Tiny sensor could transform head injury detection
Mechanical Engineering
Electrocatalytic CO2 upcycling excels under pressure
Chemical Engineering
Rethinking machine learning for frontier science

Mechanical Engineering
Falling water forms beautiful fluted films
Mechanical Engineering
Innovative strain sensor design enables extreme sensitivity
Mechanical Engineering
Turbulent flow shows surprise patterns that could help boost efficiency

Mechanical Engineering



